Kernicterus
| Kernicterus | |
|---|---|
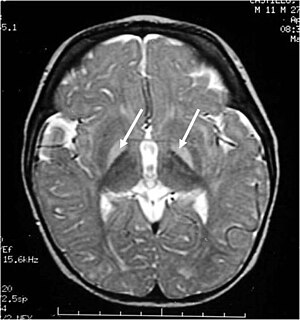 | |
| MRI of the head. Hyperintense basal ganglia lesions on T2-weighted images. | |
| Specialty | Psychiatry, Neurology, Pediatrics |
Kernicterus is a bilirubin-induced brain dysfunction. The term was coined in 1904 by Schmorl. Bilirubin is a naturally occurring substance in the body of humans and many other animals, but it is neurotoxic when its concentration in the blood is too high, a condition known as hyperbilirubinemia. Hyperbilirubinemia may cause bilirubin to accumulate in the grey matter of the central nervous system, potentially causing irreversible neurological damage. Depending on the level of exposure, the effects range from clinically unnoticeable to severe brain damage and even death.
When hyperbilirubinemia increases past a mild level, it leads to jaundice, raising the risk of progressing to kernicterus. When this happens in adults, it is usually because of liver problems. Newborns are especially vulnerable to hyperbilirubinemia-induced neurological damage, because in the earliest days of life, the still-developing liver is heavily exercised by the breakdown of fetal hemoglobin as it is replaced with adult hemoglobin and the blood brain barrier is not as developed. Mildly elevated serum bilirubin levels are common in newborns, and neonatal jaundice is not unusual, but bilirubin levels must be carefully monitored in case they start to climb, in which case more aggressive therapy is needed, usually via light therapy but sometimes even via exchange transfusion.
Contents
1 Classification
1.1 Acute bilirubin encephalopathy (ABE)
1.2 Chronic bilirubin encephalopathy (CBE)
1.3 Subtle bilirubin encephalopathy (SBE)
2 Causes
2.1 Risk factors
3 Diagnosis
4 Prevention
5 Treatment
6 References
7 External links
Classification
Acute bilirubin encephalopathy (ABE)
ABE is an acute state of elevated bilirubin in the central nervous system. Clinically, it encompasses a wide range of symptoms. These include lethargy, decreased feeding, hypotonia or hypertonia, a high-pitched cry, spasmodic torticollis, opisthotonus, setting sun sign, fever, seizures, and even death. If the bilirubin is not rapidly reduced, ABE quickly progresses to chronic bilirubin encepalopathy.
Chronic bilirubin encephalopathy (CBE)
CBE is a chronic state of severe bilirubin-induced neurological lesions. Reduction of bilirubin in this state will not reverse the sequelae. Clinically, manifestations of CBE include:
- movement disorders - athetoid cerebral palsy and or dystonia, 60% have severe motor disability (unable to walk).
- auditory dysfunction - auditory neuropathy (ANSD)
- oculomotor impairments (nystagmus, strabismus, Impaired upward or downward gaze, and/or cortical visual impairment),
- dental enamel hypoplasia/dysplasia of the deciduous teeth,
- Gastroesophageal reflux,
- impaired digestive function.
These impairments are associated with lesions in the basal ganglia, auditory nuclei of the brain stem, and oculomotor nuclei of the brain stem. Cortex and white matter are mildly involved. Cerebellum may be involved. Severe cortical involvement is uncommon.
Subtle bilirubin encephalopathy (SBE)
SBE is a chronic state of mild bilirubin-induced neurological dysfunction. Clinically, this may result in neurological, learning and movement disorders, isolated hearing loss and auditory dysfunction.
- In the past it was thought that kernicterus (KI) could cause an intellectual disability. This was assumed due to difficulty with hearing, that is rarely detected in a normal audiogram accompanied by impairments of speech. With advances in technology, this has proven to not be the case as those living with KI have repeatedly demonstrated their intelligence using Augmentative Communication devices[citation needed].
Causes
Unconjugated hyperbilirubinemia during the neonatal period describes the history of nearly all individuals who suffer from kernicterus. It is thought that the blood–brain barrier is not fully functional in neonates and therefore bilirubin is able to cross the barrier. Moreover, neonates have much higher levels of bilirubin in their blood due to:
- Although the severe anemia of erythroblastosis fetalis is usually the cause of death, many children who barely survive the anemia exhibit permanent mental impairment or damage to motor areas of the brain because of precipitation of bilirubin in the neuronal cells, causing destruction of many, a condition called kernicterus. The rapid breakdown of fetal red blood cells immediately prior to birth (and subsequent replacement by normal adult human red blood cells). This breakdown of fetal red blood cells releases large amounts of bilirubin.
- Neonates cannot metabolize and eliminate bilirubin. The sole path for bilirubin elimination is through the uridine diphosphate glucuronosyltransferase isoform 1A1 (UGT1A1) proteins that perform a (SN2 conjugation) reaction called "glucuronidation". This reaction adds a large sugar to the bilirubin and makes it more water-soluble, so more readily excreted via the urine and/or the feces. The UGT1A1 enzymes are present, but not active until several months after birth in the newborn liver. Apparently, this is a developmental compromise since the maternal liver and placenta perform glucuronidation for the fetus. In the early 1980s a late-fetal change (30 – 40 weeks of gestation) in hepatic UGT1A1 (from 0.1% to 1.0% of adult activity levels) and post-natal changes that are related to birth age not gestational age were reported. Similar development of activities to pan-specific substrates were observed except for serotonin (1A4), where adult activities were observed in fetal (16 – 25 weeks) and neonatal liver up to 10 days old. More recently, individual UGT isoform development in infants and young children, including two fetal liver samples, were analyzed and showed that pediatric levels of mRNA and protein for UGT1A1 did not differ from adults, but activities were lower. Hence, the effects of UGT1A1 developmental delay in activation have been illuminated over the last 20–30 years. The molecular mechanism(s) for activating UGT1A1 remain unknown.
- Administration of aspirin to neonates and infants. Aspirin displaces the bilirubin that was non-covalently attached to albumin in the blood stream, thus generating an increased level of free bilirubin which can cross the developing blood brain barrier. This can be life-threatening.
Bilirubin is known to accumulate in the gray matter of neurological tissue where it exerts direct neurotoxic effects. It appears that its neurotoxicity is due to mass-destruction of neurons by apoptosis and necrosis.
Risk factors
- Premature birth
- Rh incompatibility
Polycythemia - often present in neonates
Sulfonamides (e.g. co-trimoxazole) - displaces bilirubin from serum albumin
Crigler-Najjar syndrome type I- G6PD deficiency
- Bruising
Gilbert's syndrome and G6PD deficiency occurring together especially increases the risk for kernicterus.[1]
Diagnosis
Prevention
The only effective way at preventing kernicterus is to lower the serum bilirubin levels either by phototherapy or exchange transfusion. Visual inspection is never sufficient; therefore, it is best to use a bilimeter or blood test to determine a baby's risk for developing kernicterus. These numbers can then be plotted on the Bhutani nomogram.
Treatment
Currently no effective treatment exists for kernicterus. Future therapies may include neuroregeneration. A handful of patients have undergone deep brain stimulation, and experienced some benefit. Drugs such as baclofen, clonazepam, and artane are often used to manage movement disorders associated with kernicterus. Proton pump inhibitors are also used to help with reflux. Cochlear implants and hearing aids have also been known to improve the hearing loss that can come with kernicterus (auditory neuropathy - ANSD).
References
^ Cappellini MD, Di Montemuros FM, Sampietro M, Tavazzi D, Fiorelli G (1999). "The interaction between Gilbert's syndrome and G6PD deficiency influences bilirubin levels". British Journal of Haematology. 104 (4): 928–9. doi:10.1111/j.1365-2141.1999.1331a.x. PMID 10192462..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
External links
| Classification | D
|
|---|---|
| External resources |
|
- CDC’s National Center on Birth Defects and Developmental Disabilities