Polyol

Sorbitol, a common sugar alcohol, is a mild sweetener widely used in food industry.
A polyol is an organic compound containing multiple hydroxyl groups. The term "polyol" can have a slightly different meaning in food science and polymer chemistry. A molecule with more than two hydroxyl groups is a polyol, with three – a triol, and with four – a tetrol. By convention, polyols do not refer to compounds that contain other functional groups. Cellulose is a polymer with many alcohol groups, but it is not usually discussed as a polyol.
Contents
1 Sugar alcohols
2 Polymer chemistry
3 Nanoscience
4 See also
5 References
6 External links
Sugar alcohols
Sugar alcohols, a class of polyols, are commonly obtained by hydrogenation of sugars. They have the formula (CHOH)nH2, where n = 4–6.[1]
Sugar alcohols are added to foods because of their lower caloric content than sugars; however, they are also, in general, less sweet, and are often combined with high-intensity sweeteners. They are also added to chewing gum because they are not broken down by bacteria in the mouth or metabolized to acids, and thus do not contribute to tooth decay. Maltitol, sorbitol, xylitol, erythritol, and isomalt are common sugar alcohols.

The polyol pathway is a process for converting excess glucose. It is implicated in type-II diabetes.
Polymer chemistry
Polyvinyl alcohol has the formula (CH2CHOH)nH2, i.e. it has n alcohol groups where n can be in the thousands.

Structure of an idealized alkyd resin derived from the polyol glycerol (red) and phthalic anhydride.
Low molecular weight polyols are widely used in polymer chemistry, where they function as crosslinking agents. Alkyd resins for example are used in paints and in moulds for casting. They are the dominant resin or "binder" in most commercial "oil-based" coatings. Approximately 200,000 tons of alkyd resins are produced each year. They are based on linking reactive monomers with through ester formation. Polyols used in the production of commercial alkyd resins are glycerol, trimethylolpropane, and pentaerythritol.[2]
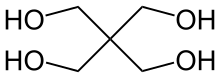
Pentaerythritol is a classic polyol used in the preparation of alkyd resins.
Polyols react with isocyanates to make polyurethanes, which find use to make mattresses, foam insulation for refrigerators and freezers, home and automotive seats, elastomeric shoe soles, fibers (e.g. Spandex), and adhesives.
Nanoscience
The preparation of metal nanoparticles often employ the "polyol method." In such cases, the polyol is usually a long chain diol such as hexadecanediol ((CH2)16(OH)2). Such diols serve as both reductants and surface-protecting agents for the nanoparticles.[3]
See also
- Cyclitol
- Polyurethane
References
^ Hubert Schiweck, Albert Bär, Roland Vogel, Eugen Schwarz, Markwart Kunz, Cécile Dusautois, Alexandre Clement, Caterine Lefranc, Bernd Lüssem, Matthias Moser, Siegfried Peters (2012). "Sugar Alcohols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheimdoi=10.1002/14356007.a25_413.pub3: Wiley-VCH.CS1 maint: Uses authors parameter (link).mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Frank N. Jones, "Alkyd Resins", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a01_409
^ Park, Jongnam; Joo, Jin; Kwon, Soon Gu; Jang, Youngjin; Hyeon, Taeghwan (2007). "Synthesis of monodisperse spherical nanocrystals". Angewandte Chemie International Edition. 46: 4630–4660. doi:10.1002/anie.200603148.CS1 maint: Uses authors parameter (link)
External links
 Media related to Polyols at Wikimedia Commons
Media related to Polyols at Wikimedia Commons