HEK 293 cells

HEK 293 cells grown for several days in standard tissue culture medium.
Human embryonic kidney 293 cells, also often referred to as HEK 293, HEK-293, 293 cells, or less precisely as HEK cells, are a specific cell line originally derived from human embryonic kidney cells grown in tissue culture. HEK 293 cells have been widely used in cell biology research for many years, because of their reliable growth and propensity for transfection. They are also used by the biotechnology industry to produce therapeutic proteins and viruses for gene therapy.
Contents
1 History
2 Applications
3 Native proteins of interest
4 References
5 External links
History

293FT cells, a variation of 293 cell
HEK 293 cells were generated in 1973 by transfection of cultures of normal human embryonic kidney cells with sheared adenovirus 5 DNA in Alex van der Eb's laboratory in Leiden, the Netherlands. The cells were obtained from a single, apparently healthy, legally aborted fetus under Dutch law; the identity of the parent and the reason for the abortion are unknown.[1] The cells were cultured by van der Eb; the transfection by adenovirus was performed by Frank Graham, a post-doc in van der Eb's lab. They were published in 1977 after Graham left Leiden for McMaster University.[2] They are called HEK since they originated in human embryonic kidney cultures, while the number 293 came from Graham's habit of numbering his experiments; the original HEK 293 cell clone was from his 293rd experiment. Graham performed the transfection a total of eight times, obtaining just one clone of cells that were cultured for several months. After presumably adapting to tissue culture, cells from this clone developed into the relatively stable HEK 293 line.
Subsequent analysis has shown that the transformation was brought about by inserting ~4.5 kilobases from the left arm of the viral genome, which became incorporated into human chromosome 19.[3]
For many years it was assumed that HEK 293 cells were generated by transformation of either a fibroblastic, endothelial or epithelial cell, all of which are abundant in kidneys. However, the original adenovirus transformation was inefficient, suggesting that the cell that finally produced the HEK 293 line may have been unusual in some fashion. Graham and coworkers provided evidence that HEK 293 cells and other human cell lines generated by adenovirus transformation of human embryonic kidney cells have many properties of immature neurons, suggesting that the adenovirus preferentially transformed a neuronal lineage cell in the original kidney culture.[4]
A comprehensive study of the genomes and transcriptomes of HEK 293 and five derivative cell lines compared the HEK 293 transcriptome with that of human kidney, adrenal, pituitary and central nervous tissue.[5] The HEK 293 pattern most closely resembled that of adrenal cells, which have many neuronal properties. Given the location of the adrenal gland (adrenal means "next to the kidney"), a few adrenal cells could plausibly have appeared in an embryonic kidney derived culture, and could be preferentially transformed by adenovirus. Adenoviruses transform neuronal lineage cells much more efficiently than typical human kidney epithelial cells.[4] An embryonic adrenal precursor cell therefore seems the most likely origin cell of the HEK 293 line. As a consequence, HEK 293 cells should not be used as an in vitro model of typical kidney cells.
HEK 293 cells have a complex karyotype, exhibiting two or more copies of each chromosome and with a modal chromosome number of 64. They are described as hypotriploid, containing less than three times the number of chromosomes of a haploid human gamete. Chromosomal abnormalities include a total of three copies of the X chromosome and four copies of chromosome 17 and chromosome 22.[5][6] The presence of multiple X chromosomes and the lack of any trace of Y chromosome derived sequence suggest that the source fetus was female.
Applications
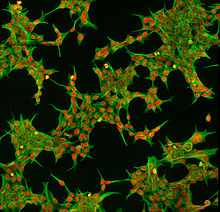
Immunofluorescent HEK 293 cells
HEK 293 cells are straightforward to grow in culture and to transfect. They have been used as hosts for gene expression. Typically, these experiments involve transfecting in a gene (or combination of genes) of interest, and then analyzing the expressed protein. The widespread use of this cell line is due to its transfectability by the various techniques, including calcium phosphate method, achieving efficiencies approaching 100%.
Examples of such experiments include:
- Effects of a drug on sodium channels[7]
- Inducible RNA interference system[8]
- Isoform-selective protein kinase C agonist[9]
- Interaction between two proteins[10]
Nuclear export signal in a protein[11]
A more specific use of HEK 293 cells is in the propagation of adenoviral vectors.[12] Viruses offer an efficient means of delivering genes into cells, which they evolved to do, and are thus of great use as experimental tools. However, as pathogens, they also present a risk to the experimenter. This danger can be avoided by the use of viruses which lack key genes, and which are thus unable to replicate after entering a cell. In order to propagate such viral vectors, a cell line that expresses the missing genes is required. Since HEK 293 cells express a number of adenoviral genes, they can be used to propagate adenoviral vectors in which these genes (typically, E1 and E3) are deleted, such as AdEasy.[13]
An important variant of this cell line is the 293T cell line. It contains the SV40 Large T-antigen that allows for episomal replication of transfected plasmids containing the SV40 origin of replication. This allows for amplification of transfected plasmids and extended temporal expression of desired gene products. Note that any similarly modified cell line can be used for this sort of work; HeLa, COS and Chinese Hamster Ovary cell are common alternatives.[citation needed] HEK 293, and especially HEK 293T, cells are commonly used for the production of various retroviral vectors.[14] Various retroviral packaging cell lines are also based on these cells.
Native proteins of interest
Depending on various conditions, the gene expression of HEK 293 cells may vary. The following proteins of interest (among many others) are commonly found in untreated HEK 293 cells:
Corticotrophin releasing factor type 1 receptor[15]
Sphingosine-1-phosphate receptors EDG1, EDG3 and EDG5[16]
Muscarinic acetylcholine receptor M3[17]
Transient receptor potential TRPC1, TRPC3, TRPC4, TRPC6[18]
References
^ Alex van der Eb. "USA FDA CTR For Biologics Evaluation and Research Vaccines and Related Biological Products Advisory Committee Meeting" (PDF). Lines 14–22: USFDA. p. 81. Retrieved August 11, 2012..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Graham FL, Smiley J, Russell WC, Nairn R (July 1977). "Characteristics of a human cell line transformed by DNA from human adenovirus type 5". J. Gen. Virol. 36 (1): 59–74. CiteSeerX 10.1.1.486.3027. doi:10.1099/0022-1317-36-1-59. PMID 886304.
^ Louis N, Evelegh C, Graham FL (July 1997). "Cloning and sequencing of the cellular-viral junctions from the human adenovirus type 5 transformed 293 cell line". Virology. 233 (2): 423–9. doi:10.1006/viro.1997.8597. PMID 9217065.
^ ab Shaw G, Morse S, Ararat M, Graham FL (June 2002). "Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells". FASEB J. 16 (8): 869–71. doi:10.1096/fj.01-0995fje. PMID 11967234.
^ ab Lin YC, Boone M, Meuris L, Lemmens I, Van Roy N, Soete A, Reumers J, Moisse M, Plaisance S, Drmanac R, Chen J, Speleman F, Lambrechts D, Van de Peer Y, Tavernier J, Callewaert N (September 2014). "Genome dynamics of the human embryonic kidney 293 lineage in response to cell biology manipulations". Nature Communications. 5 (8): 4767. Bibcode:2014NatCo...5E4767L. doi:10.1038/ncomms5767. PMC 4166678. PMID 25182477.
^ "ECACC Catalogue Entry for HEK 293". hpacultures.org.uk. ECACC. Retrieved 2012-03-18. External link in|work=(help)
^ Fredj S, Sampson KJ, Liu H, Kass RS (May 2006). "Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action". Br. J. Pharmacol. 148 (1): 16–24. doi:10.1038/sj.bjp.0706709. PMC 1617037. PMID 16520744.
^ Amar L, Desclaux M, Faucon-Biguet N, Mallet J, Vogel R (2006). "Control of small inhibitory RNA levels and RNA interference by doxycycline induced activation of a minimal RNA polymerase III promoter". Nucleic Acids Res. 34 (5): e37. doi:10.1093/nar/gkl034. PMC 1390691. PMID 16522642.
^ Kanno T, Yamamoto H, Yaguchi T, et al. (June 2006). "The linoleic acid derivative DCP-LA selectively activates PKC-epsilon, possibly binding to the phosphatidylserine binding site". J. Lipid Res. 47 (6): 1146–56. doi:10.1194/jlr.M500329-JLR200. PMID 16520488.
^ Li T, Paudel HK (March 2006). "Glycogen synthase kinase 3beta phosphorylates Alzheimer's disease-specific Ser396 of microtubule-associated protein tau by a sequential mechanism". Biochemistry. 45 (10): 3125–33. doi:10.1021/bi051634r. PMID 16519507.
^ Mustafa H, Strasser B, Rauth S, Irving RA, Wark KL (April 2006). "Identification of a functional nuclear export signal in the green fluorescent protein asFP499". Biochem. Biophys. Res. Commun. 342 (4): 1178–82. doi:10.1016/j.bbrc.2006.02.077. PMID 16516151.
^ Thomas, Philip; et al. (2005). "HEK293 cell line: A vehicle for the expression of recombinant proteins". Journal of Pharmacological and Toxicological Methods. 51 (3): 187–200. doi:10.1016/j.vascn.2004.08.014. PMID 15862464.
^ He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B (March 1998). "A simplified system for generating recombinant adenoviruses". Proc. Natl. Acad. Sci. U.S.A. 95 (5): 2509–14. Bibcode:1998PNAS...95.2509H. doi:10.1073/pnas.95.5.2509. PMC 19394. PMID 9482916.
^ Fanelli, Alex (2016). "HEK293 Cell Line: human embryonic kidney cells". Retrieved 3 December 2017.
^ Dautzenberg FM, Higelin J, Teichert U (February 2000). "Functional characterization of corticotropin-releasing factor type 1 receptor endogenously expressed in human embryonic kidney 293 cells". Eur. J. Pharmacol. 390 (1–2): 51–9. doi:10.1016/S0014-2999(99)00915-2. PMID 10708706.
^ Meyer zu Heringdorf D, Lass H, Kuchar I, et al. (March 2001). "Stimulation of intracellular sphingosine-1-phosphate production by G-protein-coupled sphingosine-1-phosphate receptors". Eur. J. Pharmacol. 414 (2–3): 145–54. doi:10.1016/S0014-2999(01)00789-0. PMID 11239914.
^ Luo J, Busillo JM, Benovic JL (April 2008). "M3 Muscarinic Acetylcholine Receptor-Mediated Signaling is Regulated by Distinct Mechanisms". Mol. Pharmacol. 74 (2): 338–47. doi:10.1124/mol.107.044750. PMID 18388243.
^ Zagranichnaya TK, Wu X, Villereal ML (August 2005). "Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells". J. Biol. Chem. 280 (33): 29559–69. doi:10.1074/jbc.M505842200. PMID 15972814.
External links
| Wikimedia Commons has media related to HEK293 cells. |
- HEK 293 Transfection and Selection Data @ Cell-culture Database
- A HEK293 Cell Database
293 Cells (CRL-1573) in the ATCC database- Transcript of FDA meeting, in which, starting page 77, van der Eb describes in detail the origin of HEK 293 cell
- 293T in the Culture Collections of Public Health England
- Cellosaurus entry for HEK 293