Pancreatic cancer
| Pancreatic cancer | |
|---|---|
 | |
| Diagram showing the position of the pancreas, behind the stomach (which is transparent in this schematic). | |
| Specialty | Oncology |
| Symptoms | Yellow skin, abdominal or back pain, unexplained weight loss, light-colored stools, dark urine, loss of appetite[1] |
| Usual onset | After 40 years old[2] |
| Risk factors | Tobacco smoking, obesity, diabetes, certain rare genetic conditions[2] |
| Diagnostic method | Medical imaging, blood tests, tissue biopsy[3][4] |
| Prevention | Not smoking, maintaining a healthy weight, low red meat diet[5] |
| Treatment | Surgery, radiotherapy, chemotherapy, palliative care[1] |
| Prognosis | Five year survival rate 5%[6][7] |
| Frequency | 393,800 (2015)[8] |
| Deaths | 411,600 (2015)[9] |
Pancreatic cancer arises when cells in the pancreas, a glandular organ behind the stomach, begin to multiply out of control and form a mass. These cancerous cells have the ability to invade other parts of the body.[10] There are a number of types of pancreatic cancer.[6] The most common, pancreatic adenocarcinoma, accounts for about 85% of cases, and the term "pancreatic cancer" is sometimes used to refer only to that type.[6] These adenocarcinomas start within the part of the pancreas which makes digestive enzymes.[6] Several other types of cancer, which collectively represent the majority of the non-adenocarcinomas, can also arise from these cells.[6] One to two percent of cases of pancreatic cancer are neuroendocrine tumors, which arise from the hormone-producing cells of the pancreas.[6] These are generally less aggressive than pancreatic adenocarcinoma.[6]
Signs and symptoms of the most common form of pancreatic cancer may include yellow skin, abdominal or back pain, unexplained weight loss, light-colored stools, dark urine and loss of appetite.[1] There are usually no symptoms in the disease's early stages, and symptoms that are specific enough to suggest pancreatic cancer typically do not develop until the disease has reached an advanced stage.[1][2] By the time of diagnosis, pancreatic cancer has often spread to other parts of the body.[6][11]
Pancreatic cancer rarely occurs before the age of 40, and more than half of cases of pancreatic adenocarcinoma occur in those over 70.[2] Risk factors for pancreatic cancer include tobacco smoking, obesity, diabetes, and certain rare genetic conditions.[2] About 25% of cases are linked to smoking,[3] and 5–10% are linked to inherited genes.[2] Pancreatic cancer is usually diagnosed by a combination of medical imaging techniques such as ultrasound or computed tomography, blood tests, and examination of tissue samples (biopsy).[3][4] The disease is divided into stages, from early (stage I) to late (stage IV).[11]Screening the general population has not been found to be effective.[12][13]
The risk of developing pancreatic cancer is lower among non-smokers, and people who maintain a healthy weight and limit their consumption of red or processed meat.[5] A smoker's chance of developing the disease decreases if they stop smoking, and almost returns to that of the rest of the population after 20 years.[6] Pancreatic cancer can be treated with surgery, radiotherapy, chemotherapy, palliative care, or a combination of these.[1] Treatment options are partly based on the cancer stage.[1] Surgery is the only treatment that can cure pancreatic adenocarcinoma,[11] and may also be done to improve quality of life without the potential for cure.[1][11]Pain management and medications to improve digestion are sometimes needed.[11] Early palliative care is recommended even for those receiving treatment that aims for a cure.[14][15]
In 2015, pancreatic cancers of all types resulted in 411,600 deaths globally.[9] Pancreatic cancer is the fifth most common cause of death from cancer in the United Kingdom,[16] and the fourth most common in the United States.[17][18] The disease occurs most often in the developed world, where about 70% of the new cases in 2012 originated.[6] Pancreatic adenocarcinoma typically has a very poor prognosis: after diagnosis, 25% of people survive one year and 5% live for five years.[6][7] For cancers diagnosed early, the five-year survival rate rises to about 20%.[19] Neuroendocrine cancers have better outcomes; at five years from diagnosis, 65% of those diagnosed are living, though survival varies considerably depending on the type of tumor.[6]
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 ul{display:none}
Contents
1 Types
1.1 Exocrine cancers
1.2 Neuroendocrine
2 Signs and symptoms
2.1 Other findings
2.2 Symptoms of spread
3 Risk factors
3.1 Alcohol
4 Pathophysiology
4.1 Precancer
4.2 Invasive cancer
4.3 PanNETs
5 Diagnosis
6 Staging
6.1 Exocrine cancers
6.2 PanNETs
7 Prevention and screening
8 Management
8.1 Exocrine cancer
8.1.1 Surgery
8.1.2 Chemotherapy
8.1.3 Radiotherapy
8.2 PanNETs
8.3 Palliative care
9 Outcomes
10 Distribution
10.1 PanNETs
11 History
12 Research directions
13 See also
14 References
15 External links
Types

The pancreas has multiple functions, served by the endocrine cells in the islets of Langerhans and the exocrine acinar cells. Pancreatic cancer may arise from any of these and disrupt any of their functions.
The many types of pancreatic cancer can be divided into two general groups. The vast majority of cases (about 95%) occur in the part of the pancreas which produces digestive enzymes, known as the exocrine component. There are several sub-types of exocrine pancreatic cancers, but their diagnosis and treatment have much in common. The small minority of cancers that arise in the hormone-producing (endocrine) tissue of the pancreas have different clinical characteristics and are called pancreatic neuroendocrine tumors, sometimes abbreviated as "PanNETs". Both groups occur mainly (but not exclusively) in people over 40, and are slightly more common in men, but some rare sub-types mainly occur in women or children.[20][21]
Exocrine cancers
The exocrine group is dominated by pancreatic adenocarcinoma (variations of this name may add "invasive" and "ductal"), which is by far the most common type, representing about 85% of all pancreatic cancers.[2] Nearly all these start in the ducts of the pancreas, as pancreatic ductal adenocarcinoma (PDAC).[22] This is despite the fact that the tissue from which it arises – the pancreatic ductal epithelium – represents less than 10% of the pancreas by cell volume, because it constitutes only the ducts (an extensive but capillary-like duct-system fanning out) within the pancreas.[23] This cancer originates in the ducts that carry secretions (such as enzymes and bicarbonate) away from the pancreas. About 60–70% of adenocarcinomas occur in the head of the pancreas.[2]
The next most common type, acinar cell carcinoma of the pancreas, arises in the clusters of cells that produce these enzymes, and represents 5% of exocrine pancreas cancers.[24] Like the 'functioning' endocrine cancers described below, acinar cell carcinomas may cause over-production of certain molecules, in this case digestive enzymes, which may cause symptoms such as skin rashes and joint pain.
Cystadenocarcinomas account for 1% of pancreatic cancers, and they have a better prognosis than the other exocrine types.[24]
Pancreatoblastoma is a rare form, mostly occurring in childhood, and with a relatively good prognosis. Other exocrine cancers include adenosquamous carcinomas, signet ring cell carcinomas, hepatoid carcinomas, colloid carcinomas, undifferentiated carcinomas, and undifferentiated carcinomas with osteoclast-like giant cells. Solid pseudopapillary tumor is a rare low-grade neoplasm that mainly affects younger women, and generally has a very good prognosis.[2][25]
Pancreatic mucinous cystic neoplasms are a broad group of pancreas tumors that have varying malignant potential. They are being detected at a greatly increased rate as CT scans become more powerful and common, and discussion continues as how best to assess and treat them, given that many are benign.[26]
Neuroendocrine
The small minority of tumors that arise elsewhere in the pancreas are mainly pancreatic neuroendocrine tumors (PanNETs).[27]Neuroendocrine tumors (NETs) are a diverse group of benign or malignant tumors that arise from the body's neuroendocrine cells, which are responsible for integrating the nervous and endocrine systems. NETs can start in most organs of the body, including the pancreas, where the various malignant types are all considered to be rare. PanNETs are grouped into 'functioning' and 'non-functioning' types, depending on the degree to which they produce hormones. The functioning types secrete hormones such as insulin, gastrin, and glucagon into the bloodstream, often in large quantities, giving rise to serious symptoms such as low blood sugar, but also favoring relatively early detection. The most common functioning PanNETs are insulinomas and gastrinomas, named after the hormones they secrete. The non-functioning types do not secrete hormones in a sufficient quantity to give rise to overt clinical symptoms. For this reason, non-functioning PanNETs are often diagnosed only after the cancer has spread to other parts of the body.[28]
As with other neuroendocrine tumors, the history of the terminology and classification of PanNETs is complex.[27] PanNETs are sometimes called "islet cell cancers",[29] even though it is now known that they do not actually arise from islet cells as previously thought.[28]
Signs and symptoms

Jaundice can be a symptom, due to biliary obstruction from a pancreatic tumor.
Since pancreatic cancer usually does not cause recognizable symptoms in its early stages, the disease is typically not diagnosed until it has spread beyond the pancreas itself.[4] This is one of the main reasons for the generally poor survival rates. Exceptions to this are the functioning PanNETs, where over-production of various active hormones can give rise to symptoms (which depend on the type of hormone).[30]
Bearing in mind that the disease is rarely diagnosed before the age of 40, common symptoms of pancreatic adenocarcinoma occurring before diagnosis include:
Pain in the upper abdomen or back, often spreading from around the stomach to the back. The location of the pain can indicate the part of the pancreas where a tumor is located. The pain may be worse at night and may increase over time to become severe and unremitting.[24] It may be slightly relieved by bending forward. In the UK, about half of new cases of pancreatic cancer are diagnosed following a visit to a hospital emergency department for pain or jaundice. In up to two-thirds of people abdominal pain is the main symptom, for 46% of the total accompanied by jaundice, with 13% having jaundice without pain.[11]
Jaundice, a yellow tint to the whites of the eyes or skin, with or without pain, and possibly in combination with darkened urine. This results when a cancer in the head of the pancreas obstructs the common bile duct as it runs through the pancreas.[31]
Unexplained weight loss, either from loss of appetite, or loss of exocrine function resulting in poor digestion.[11]
- The tumor may compress neighboring organs, disrupting digestive processes and making it difficult for the stomach to empty, which may cause nausea and a feeling of fullness. The undigested fat leads to foul-smelling, fatty feces that are difficult to flush away.[11]Constipation is common.[32]
- At least 50% of people with pancreatic adenocarcinoma have diabetes at the time of diagnosis.[2] While long-standing diabetes is a known risk factor for pancreatic cancer (see Risk factors), the cancer can itself cause diabetes, in which case recent onset of diabetes could be considered an early sign of the disease.[33] People over 50 who develop diabetes have eight times the usual risk of developing pancreatic adenocarcinoma within three years, after which the relative risk declines.[11]
Other findings
Trousseau's syndrome, in which blood clots form spontaneously in the portal blood vessels, the deep veins of the extremities, or the superficial veins anywhere on the body, may be associated with pancreatic cancer, and is found in about 10% of cases.[3]
Clinical depression has been reported in association with pancreatic cancer in some 10–20% of cases, and can be a hindrance to optimal management. The depression sometimes appears before the diagnosis of cancer, suggesting that it may be brought on by the biology of the disease.[3]
Other common manifestations of the disease include: weakness and tiring easily; dry mouth; sleep problems; and a palpable abdominal mass.[32]
Symptoms of spread

Cross section of a human liver, at autopsy, showing multiple large pale tumor deposits, that are secondary tumors derived from pancreatic cancer
The spread of pancreatic cancer to other organs (metastasis) may also cause symptoms. Typically, pancreatic adenocarcinoma first spreads to nearby lymph nodes, and later to the liver or to the peritoneal cavity, large intestine or lungs.[3] It is uncommon for it to spread to the bones or brain.[34]
Cancers in the pancreas may also be secondary cancers that have spread from other parts of the body. This is uncommon, found in only about 2% of cases of pancreatic cancer. Kidney cancer is by far the most common cancer to spread to the pancreas, followed by colorectal cancer, and then cancers of the skin, breast, and lung. Surgery may be performed on the pancreas in such cases, whether in hope of a cure or to alleviate symptoms.[35]
Risk factors
Risk factors for pancreatic adenocarcinoma include:[2][6][11][36]
- Age, sex, and ethnicity; the risk of developing pancreatic cancer increases with age. Most cases occur after age 65,[6] while cases before age 40 are uncommon. The disease is slightly more common in men than women, and in the United States is over 1.5 times more common in African Americans, though incidence in Africa is low.[6]
Cigarette smoking is the best-established avoidable risk factor for pancreatic cancer, approximately doubling risk among long-term smokers, the risk increasing with the number of cigarettes smoked and the years of smoking. The risk declines slowly after smoking cessation, taking some 20 years to return to almost that of non-smokers.[37]
Obesity; a BMI greater than 35 increases relative risk by about half.[11]
- Family history; 5–10% of pancreatic cancer cases have an inherited component, where people have a family history of pancreatic cancer.[2][38] The risk escalates greatly if more than one first-degree relative had the disease, and more modestly if they developed it before the age of 50.[4] Most of the genes involved have not been identified.[2][39]Hereditary pancreatitis gives a greatly increased lifetime risk of pancreatic cancer of 30–40% to the age of 70.[3] Screening for early pancreatic cancer may be offered to individuals with hereditary pancreatitis on a research basis.[40] Some people may choose to have their pancreas surgically removed to prevent cancer developing in the future.[3]
- Pancreatic cancer has been associated with the following other rare hereditary syndromes: Peutz–Jeghers syndrome due to mutations in the STK11 tumor suppressor gene (very rare, but a very strong risk factor); dysplastic nevus syndrome (or familial atypical multiple mole and melanoma syndrome, FAMMM-PC) due to mutations in the CDKN2A tumor suppressor gene; autosomal recessive ataxia-telangiectasia and autosomal dominantly inherited mutations in the BRCA2 gene and PALB2 gene; hereditary non-polyposis colon cancer (Lynch syndrome); and familial adenomatous polyposis. PanNETs have been associated with multiple endocrine neoplasia type 1 (MEN1) and von Hippel Lindau syndromes.[2][3][4]
Chronic pancreatitis appears to almost triple risk, and as with diabetes, new-onset pancreatitis may be a symptom of a tumor.[3] The risk of pancreatic cancer in individuals with familial pancreatitis is particularly high.[3][39]
Diabetes mellitus is a risk factor for pancreatic cancer and (as noted in the Signs and symptoms section) new-onset diabetes may also be an early sign of the disease. People who have been diagnosed with Type 2 diabetes for longer than ten years may have a 50% increased risk, as compared with individuals without diabetes.[3]
- Specific types of food (as distinct from obesity) have not been clearly shown to increase the risk of pancreatic cancer.[2][41] Dietary factors for which there is some evidence of slightly increased risk include processed meat, red meat, and meat cooked at very high temperatures (e.g. by frying, broiling or barbecuing).[41][42]
Alcohol
Drinking alcohol excessively is a major cause of chronic pancreatitis, which in turn predisposes to pancreatic cancer. However, considerable research has failed to firmly establish alcohol consumption as a direct risk factor for pancreatic cancer. Overall, the association is consistently weak and the majority of studies have found no association, with smoking a strong confounding factor. The evidence is stronger for a link with heavy drinking, of at least six drinks per day.[3][43]
Pathophysiology

Micrograph of pancreatic ductal adenocarcinoma (the most common type of pancreatic cancer). H&E stain
Precancer

Micrographs of normal pancreas, pancreatic intraepithelial neoplasia (precursors to pancreatic carcinoma) and pancreatic carcinoma. H&E stain
Exocrine cancers are thought to arise from several types of precancerous lesions within the pancreas. But these lesions do not always progress to cancer, and the increased numbers detected as a by-product of the increasing use of CT scans for other reasons are not all treated.[3] Apart from pancreatic serous cystadenomas (SCNs), which are almost always benign, four types of precancerous lesion are recognized.
The first is pancreatic intraepithelial neoplasia. These lesions are microscopic abnormalities in the pancreas and are often found in autopsies of people with no diagnosed cancer. These lesions may progress from low to high grade and then to a tumor. More than 90% of cases at all grades carry a faulty KRAS gene, while in grades 2 and 3 damage to three further genes – CDKN2A (p16), p53 and SMAD4 – are increasingly often found.[2]
A second type are the intraductal papillary mucinous neoplasms (IPMNs). These are macroscopic lesions, which are found in about 2% of all adults. This rate rises to ~10% by age 70. These lesions have about a 25% risk of developing into invasive cancer. They may have KRAS gene mutations (~40–65% of cases) and in the GNAS Gs alpha subunit and RNF43, affecting the Wnt signaling pathway.[2] Even if removed surgically, there remains a considerably increased risk of pancreatic cancer developing subsequently.[3]
The third type, pancreatic mucinous cystic neoplasms (MCNs) mainly occur in women, and may remain benign or progress to cancer.[44] If these lesions become large, cause symptoms, or have suspicious features, they can usually be successfully removed by surgery.[3]
A fourth type of cancer that arises in the pancreas is the intraductal tubulopapillary neoplasm. This type was recognised by the WHO in 2010 and constitutes about 1–3% of all pancreatic neoplasms. Mean age at diagnosis is 61 years (range 35–78 years). About 50% of these lesions become invasive. Diagnosis depends on histology as these lesions are very difficult to differentiate from other lesions on either clinical or radiological grounds.[45]
Invasive cancer
The genetic events found in ductal adenocarcinoma have been well characterized, and complete exome sequencing has been done for the common types of tumor. Four genes have each been found to be mutated in the majority of adenocarcinomas: KRAS (in 95% of cases), CDKN2A (also in 95%), TP53 (75%), and SMAD4 (55%). The last of these are especially associated with a poor prognosis.[3]SWI/SNF mutations/deletions occur in about 10–15% of the adenocarcinomas.[2] The genetic alterations in several other types of pancreatic cancer and precancerous lesions have also been researched.[3] Transcriptomics analyses and mRNA sequencing for the common forms of pancreatic cancer have found that 75% of human genes are expressed in the tumors, with some 200 genes more specifically expressed in pancreatic cancer as compared to other tumor types.[46][47]
PanNETs
The genes often found mutated in PanNETs are different from those in exocrine pancreatic cancer.[48] For example, KRAS mutation is normally absent. Instead, hereditary MEN1 gene mutations give rise to MEN1 syndrome, in which primary tumors occur in two or more endocrine glands. About 40–70% of people born with a MEN1 mutation eventually develop a PanNet.[49] Other genes that are frequently mutated include DAXX, mTOR and ATRX.[28]
Diagnosis
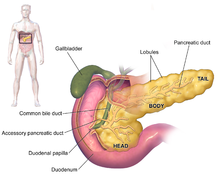
The head, body and tail of the pancreas. The stomach is faded out in this image to show the entire pancreas, of which the body and tail lie behind the stomach, and the neck partially behind.

Axial CT image with IV contrast and added color. Cross lines towards top left surround a macrocystic adenocarcinoma of the pancreatic head.

Abdominal ultrasonography of pancreatic cancer (presumably adenocarcinoma), with a dilated pancreatic duct to the right.
The symptoms of pancreatic adenocarcinoma do not usually appear in the disease's early stages, and are individually not distinctive to the disease.[3][11][31] The symptoms at diagnosis vary according to the location of the cancer in the pancreas, which anatomists divide (from left to right on most diagrams) into the thick head, the neck, and the tapering body, ending in the tail.
Regardless of a tumor's location, the most common symptom is unexplained weight loss, which may be considerable. A large minority (between 35% and 47%) of people diagnosed with the disease will have had nausea, vomiting or a feeling of weakness. Tumors in the head of the pancreas typically also cause jaundice, pain, loss of appetite, dark urine, and light-colored stools. Tumors in the body and tail typically also cause pain.[31]
People sometimes have recent onset of atypical type 2 diabetes that is difficult to control, a history of recent but unexplained blood vessel inflammation caused by blood clots (thrombophlebitis) known as Trousseau sign, or a previous attack of pancreatitis.[31] A doctor may suspect pancreatic cancer when the onset of diabetes in someone over 50 years old is accompanied by typical symptoms such as unexplained weight loss, persistent abdominal or back pain, indigestion, vomiting, or fatty feces.[11] Jaundice accompanied by a painlessly swollen gallbladder (known as Courvoisier's sign) may also raise suspicion, and can help differentiate pancreatic cancer from gallstones.[50]
Medical imaging techniques, such as computed tomography (CT scan) and endoscopic ultrasound (EUS) are used both to confirm the diagnosis and to help decide whether the tumor can be surgically removed (its "resectability").[11] On contrast CT scan, pancreatic cancer typically shows a gradually increasing radiocontrast uptake, rather than a fast washout as seen in a normal pancreas or a delayed washout as seen in chronic pancreatitis.[51]Magnetic resonance imaging and positron emission tomography may also be used,[2] and magnetic resonance cholangiopancreatography may be useful in some cases.[31]Abdominal ultrasound is less sensitive and will miss small tumors, but can identify cancers that have spread to the liver and build-up of fluid in the peritoneal cavity (ascites).[11] It may be used for a quick and cheap first examination before other techniques.[52]
A biopsy by fine needle aspiration, often guided by endoscopic ultrasound, may be used where there is uncertainty over the diagnosis, but a histologic diagnosis is not usually required for removal of the tumor by surgery to go ahead.[11]
Liver function tests can show a combination of results indicative of bile duct obstruction (raised conjugated bilirubin, γ-glutamyl transpeptidase and alkaline phosphatase levels). CA19-9 (carbohydrate antigen 19.9) is a tumor marker that is frequently elevated in pancreatic cancer. However, it lacks sensitivity and specificity, not least because 5% of people lack the Lewis (a) antigen and cannot produce CA19-9. It has a sensitivity of 80% and specificity of 73% in detecting pancreatic adenocarcinoma, and is used for following known cases rather than diagnosis.[2][11]
The most common form of pancreatic cancer (adenocarcinoma) is typically characterized by moderately to poorly differentiated glandular structures on microscopic examination. There is typically considerable desmoplasia or formation of a dense fibrous stroma or structural tissue consisting of a range of cell types (including myofibroblasts, macrophages, lymphocytes and mast cells) and deposited material (such as type I collagen and hyaluronic acid). This creates a tumor microenvironment that is short of blood vessels (hypovascular) and so of oxygen (tumor hypoxia).[2] It is thought that this prevents many chemotherapy drugs from reaching the tumor, as one factor making the cancer especially hard to treat.[2][3]
Staging
Exocrine cancers
Pancreatic cancer is usually staged following a CT scan.[31] The most widely used cancer staging system for pancreatic cancer is the one formulated by the American Joint Committee on Cancer (AJCC) together with the Union for International Cancer Control (UICC). The AJCC-UICC staging system designates four main overall stages, ranging from early to advanced disease, based on TNM classification of Tumor size, spread to lymph Nodes, and Metastasis.[53]
To help decide treatment, the tumors are also divided into three broader categories based on whether surgical removal seems possible: in this way, tumors are judged to be "resectable", "borderline resectable", or "unresectable".[54] When the disease is still in an early stage (AJCC-UICC stages I and II), without spread to large blood vessels or distant organs such as the liver or lungs, surgical resection of the tumor can normally be performed, if the patient is willing to undergo this major operation and is thought to be sufficiently fit.[11] The AJCC-UICC staging system allows distinction between stage III tumors that are judged to be "borderline resectable" (where surgery is technically feasible because the celiac axis and superior mesenteric artery are still free) and those that are "unresectable" (due to more locally advanced disease); in terms of the more detailed TNM classification, these two groups correspond to T3 and T4 respectively.[3]
- Pancreatic cancer staging (TNM classification)

Stage T1 pancreatic cancer

Stage T2 pancreatic cancer
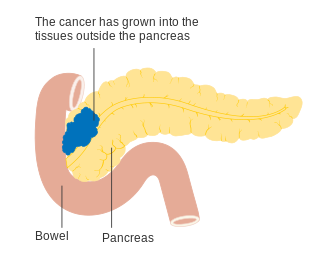
Stage T3 pancreatic cancer
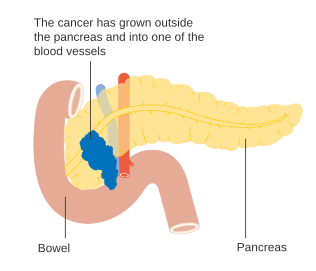
Stage T4 pancreatic cancer

Pancreatic cancer in nearby lymph nodes – Stage N1

Pancreatic cancer metastasized – stage M1
Locally advanced adenocarcinomas have spread into neighboring organs, which may be any of the following (in roughly decreasing order of frequency): the duodenum, stomach, transverse colon, spleen, adrenal gland, or kidney. Very often they also spread to the important blood or lymphatic vessels and nerves that run close to the pancreas, making surgery far more difficult. Typical sites for metastatic spread (stage IV disease) are the liver, peritoneal cavity and lungs, all of which occur in 50% or more of fully advanced cases.[55]
PanNETs
The 2010 WHO classification of tumors of the digestive system grades all the pancreatic neuroendocrine tumors (PanNETs) into three categories, based on their degree of cellular differentiation (from "NET G1" through to the poorly differentiated "NET G3").[21] The U.S. National Comprehensive Cancer Network recommends use of the same AJCC-UICC staging system as pancreatic adenocarcinoma.[56]:52 Using this scheme, the stage-by-stage outcomes for PanNETs are dissimilar to those of the exocrine cancers.[57] A different TNM system for PanNETs has been proposed by the European Neuroendocrine Tumor Society.[21]
Prevention and screening
Apart from not smoking, the American Cancer Society recommends keeping a healthy weight, and increasing consumption of fruits, vegetables, and whole grains, while decreasing consumption of red and processed meat, although there is no consistent evidence this will prevent or reduce pancreatic cancer specifically.[58] A 2014 review of research concluded that there was evidence that consumption of citrus fruits and curcumin reduced risk of pancreatic cancer, while there was possibly a beneficial effect from whole grains, folate, selenium, and non-fried fish.[43]
In the general population, screening of large groups is not currently considered effective, although newer techniques, and the screening of tightly targeted groups, are being evaluated.[59][60] Nevertheless, regular screening with endoscopic ultrasound and MRI/CT imaging is recommended for those at high risk from inherited genetics.[4][52][60][61]
Management
Exocrine cancer
A key assessment that is made after diagnosis is whether surgical removal of the tumor is possible (see Staging), as this is the only cure for this cancer. Whether or not surgical resection can be offered depends on how much the cancer has spread. The exact location of the tumor is also a significant factor, and CT can show how it relates to the major blood vessels passing close to the pancreas. The general health of the person must also be assessed, though age in itself is not an obstacle to surgery.[3]
Chemotherapy and, to a lesser extent, radiotherapy are likely to be offered to most people, whether or not surgery is possible. Specialists advise that the management of pancreatic cancer should be in the hands of a multidisciplinary team including specialists in several aspects of oncology, and is, therefore, best conducted in larger centers.[2][3]
Surgery

Parts of the body removed in Whipple's operation
Surgery with the intention of a cure is only possible in around one-fifth (20%) of new cases.[11] Although CT scans help, in practice it can be difficult to determine whether the tumor can be fully removed (its "resectability"), and it may only become apparent during surgery that it is not possible to successfully remove the tumor without damaging other vital tissues. Whether or not surgical resection can be offered depends on various factors, including the precise extent of local anatomical adjacency to, or involvement of, the venous or arterial blood vessels,[2] as well as surgical expertise and a careful consideration of projected post-operative recovery.[62][63] The age of the person is not in itself a reason not to operate, but their general performance status needs to be adequate for a major operation.[11]
One particular feature that is evaluated is the encouraging presence, or discouraging absence, of a clear layer or plane of fat creating a barrier between the tumor and the vessels.[3] Traditionally, an assessment is made of the tumor's proximity to major venous or arterial vessels, in terms of "abutment" (defined as the tumor touching no more than half a blood vessel's circumference without any fat to separate it), "encasement" (when the tumor encloses most of the vessel's circumference), or full vessel involvement.[64]:22 A resection that includes encased sections of blood vessels may be possible in some cases,[65][66] particularly if preliminary neoadjuvant therapy is feasible,[67][68][69] using chemotherapy[63][64]:36[70] and/or radiotherapy.[64]:29–30
Even when the operation appears to have been successful, cancerous cells are often found around the edges ("margins") of the removed tissue, when a pathologist examines them microscopically (this will always be done), indicating the cancer has not been entirely removed.[2] Furthermore, cancer stem cells are usually not evident microscopically, and if they are present they may continue to develop and spread.[71][72] An exploratory laparoscopy (a small, camera-guided surgical procedure) may therefore be performed to gain a clearer idea of the outcome of a full operation.[73]

How the pancreas and bowel are joined back together after a Whipple's operation
For cancers involving the head of the pancreas, the Whipple procedure is the most commonly attempted curative surgical treatment. This is a major operation which involves removing the pancreatic head and the curve of the duodenum together ("pancreato-duodenectomy"), making a bypass for food from the stomach to the jejunum ("gastro-jejunostomy") and attaching a loop of jejunum to the cystic duct to drain bile ("cholecysto-jejunostomy"). It can be performed only if the person is likely to survive major surgery and if the cancer is localized without invading local structures or metastasizing. It can, therefore, be performed only in a minority of cases. Cancers of the tail of the pancreas can be resected using a procedure known as a distal pancreatectomy, which often also entails removal of the spleen.[2][3] Nowadays, this can often be done using minimally invasive surgery.[2][3]
Although curative surgery no longer entails the very high death rates that occurred until the 1980s, a high proportion of people (about 30–45%) still have to be treated for a post-operative sickness that is not caused by the cancer itself. The most common complication of surgery is difficulty in emptying the stomach.[3] Certain more limited surgical procedures may also be used to ease symptoms (see Palliative care): for instance, if the cancer is invading or compressing the duodenum or colon. In such cases, bypass surgery might overcome the obstruction and improve quality of life but is not intended as a cure.[11]
Chemotherapy
After surgery, adjuvant chemotherapy with gemcitabine or 5-FU can be offered if the person is sufficiently fit, after a recovery period of one to two months.[4][52] In people not suitable for curative surgery, chemotherapy may be used to extend life or improve its quality.[3] Before surgery, neoadjuvant chemotherapy or chemoradiotherapy may be used in cases that are considered to be "borderline resectable" (see Staging) in order to reduce the cancer to a level where surgery could be beneficial. In other cases neoadjuvant therapy remains controversial, because it delays surgery.[3][4][74]
Gemcitabine was approved by the United States Food and Drug Administration (FDA) in 1997, after a clinical trial reported improvements in quality of life and a 5-week improvement in median survival duration in people with advanced pancreatic cancer.[75] This was the first chemotherapy drug approved by the FDA primarily for a nonsurvival clinical trial endpoint.[76] Chemotherapy using gemcitabine alone was the standard for about a decade, as a number of trials testing it in combination with other drugs failed to demonstrate significantly better outcomes. However, the combination of gemcitabine with erlotinib was found to increase survival modestly, and erlotinib was licensed by the FDA for use in pancreatic cancer in 2005.[77]
The FOLFIRINOX chemotherapy regimen using four drugs was found more effective than gemcitabine, but with substantial side effects, and is thus only suitable for people with good performance status. This is also true of protein-bound paclitaxel (nab-paclitaxel), which was licensed by the FDA in 2013 for use with gemcitabine in pancreas cancer.[78] By the end of 2013, both FOLFIRINOX and nab-paclitaxel with gemcitabine were regarded as good choices for those able to tolerate the side-effects, and gemcitabine remained an effective option for those who were not. A head-to-head trial between the two new options is awaited, and trials investigating other variations continue. However, the changes of the last few years have only increased survival times by a few months.[75] Clinical trials are often conducted for novel adjuvant therapies.[4]
Radiotherapy
The role of radiotherapy as an auxiliary (adjuvant) treatment after potentially curative surgery has been controversial since the 1980s.[3] The European Society for Medical Oncology recommends that adjuvant radiotherapy should only be used for people enrolled in clinical trials.[52] However, there is a continuing tendency for clinicians in the US to be more ready to use adjuvant radiotherapy than those in Europe. Many clinical trials have tested a variety of treatment combinations since the 1980s, but have failed to settle the matter conclusively.[3][4]
Radiotherapy may form part of treatment to attempt to shrink a tumor to a resectable state, but its use on unresectable tumors remains controversial as there are conflicting results from clinical trials. The preliminary results of one trial, presented in 2013, "markedly reduced enthusiasm" for its use on locally advanced tumors.[2]
PanNETs
Treatment of PanNETs, including the less common malignant types, may include a number of approaches.[56][79][80][81] Some small tumors of less than 1 cm. that are identified incidentally, for example on a CT scan performed for other purposes, may be followed by watchful waiting.[56] This depends on the assessed risk of surgery which is influenced by the site of the tumor and the presence of other medical problems.[56] Tumors within the pancreas only (localized tumors), or with limited metastases, for example to the liver, may be removed by surgery. The type of surgery depends on the tumor location, and the degree of spread to lymph nodes.[21]
For localized tumors, the surgical procedure may be much less extensive than the types of surgery used to treat pancreatic adenocarcinoma described above, but otherwise surgical procedures are similar to those for exocrine tumors. The range of possible outcomes varies greatly; some types have a very high survival rate after surgery while others have a poor outlook. As all this group are rare, guidelines emphasize that treatment should be undertaken in a specialized center.[21][28] Use of liver transplantation may be considered in certain cases of liver metastasis.[82]
For functioning tumors, the somatostatin analog class of medications, such as octreotide, can reduce the excessive production of hormones.[21]Lanreotide can slow tumor growth.[83] If the tumor is not amenable to surgical removal and is causing symptoms, targeted therapy with everolimus or sunitinib can reduce symptoms and slow progression of the disease.[28][84][85] Standard cytotoxic chemotherapy is generally not very effective for PanNETs, but may be used when other drug treatments fail to prevent the disease from progressing,[28][86] or in poorly differentiated PanNET cancers.[87]
Radiation therapy is occasionally used if there is pain due to anatomic extension, such as metastasis to bone. Some PanNETs absorb specific peptides or hormones, and these PanNETs may respond to nuclear medicine therapy with radiolabeled peptides or hormones such as iobenguane (iodine-131-MIBG).[88][89][90][91]Radiofrequency ablation (RFA), cryoablation, and hepatic artery embolization may also be used.[92][93]
Palliative care
Palliative care is medical care which focuses on treatment of symptoms from serious illness, such as cancer, and improving quality of life.[94] Because pancreatic adenocarcinoma is usually diagnosed after it has progressed to an advanced stage, palliative care as a treatment of symptoms is often the only treatment possible.[95]
Palliative care focuses not on treating the underlying cancer, but on treating symptoms such as pain or nausea, and can assist in decision-making, including when or if hospice care will be beneficial.[96] Pain can be managed with medications such as opioids or through procedural intervention, by a nerve block on the celiac plexus (CPB). This alters or, depending on the technique used, destroys the nerves that transmit pain from the abdomen. CPB is a safe and effective way to reduce the pain, which generally reduces the need to use opioid painkillers, which have significant negative side effects.[3][97]
Other symptoms or complications that can be treated with palliative surgery are obstruction by the tumor of the intestines or bile ducts. For the latter, which occurs in well over half of cases, a small metal tube called a stent may be inserted by endoscope to keep the ducts draining.[31] Palliative care can also help treat depression that often comes with the diagnosis of pancreatic cancer.[3]
Both surgery and advanced inoperable tumors often lead to digestive system disorders from a lack of the exocrine products of the pancreas (exocrine insufficiency). These can be treated by taking pancreatin which contains manufactured pancreatic enzymes, and is best taken with food.[11] Difficulty in emptying the stomach (delayed gastric emptying) is common and can be a serious problem, involving hospitalization. Treatment may involve a variety of approaches, including draining the stomach by nasogastric aspiration and drugs called proton-pump inhibitors or H2 antagonists, which both reduce production of gastric acid.[11] Medications like metoclopramide can also be used to clear stomach contents.
Outcomes
| Clinical stage | Five-year survival (%) – U.S., diagnoses 1992–98 | |
|---|---|---|
| Exocrine pancreatic cancer | Neuroendocrine treated with surgery | |
| IA / I | 14 | 61 |
| IB | 12 | |
| IIA / II | 7 | 52 |
| IIB | 5 | |
| III | 3 | 41 |
| IV | 1 | 16 |
Pancreatic adenocarcinoma and the other less common exocrine cancers have a very poor prognosis, as they are normally diagnosed at a late stage when the cancer is already locally advanced or has spread to other parts of the body.[2] Outcomes are much better for PanNETs: many are benign and completely without clinical symptoms, and even those cases not treatable with surgery have an average five-year survival rate of 16%,[54] although the outlook varies considerably according to the type.[30]
For locally advanced and metastatic pancreatic adenocarcinomas, which together represent over 80% of cases, numerous recent trials comparing chemotherapy regimes have shown increased survival times, but not to more than one year.[2][75] Overall five-year survival for pancreatic cancer in the US has improved from 2% in cases diagnosed in 1975–77, and 4% in 1987–89 diagnoses, to 6% in 2003–09.[98] In the less than 20% of cases of pancreatic adenocarcinoma with a diagnosis of a localized and small cancerous growth (less than 2 cm in Stage T1), about 20% of Americans survive to five years.[19]
About 1500 genes are linked to outcomes in pancreatic adenocarcinoma. These include both unfavorable genes, where high expression is related to poor outcome, for example C-Met and MUC-1, and favorable genes where high expression is associated with better survival, for example the transcription factor PELP1.[46][47]
Distribution

Deaths from pancreatic cancer per million persons in 2012 .mw-parser-output .refbegin{font-size:90%;margin-bottom:0.5em}.mw-parser-output .refbegin-hanging-indents>ul{list-style-type:none;margin-left:0}.mw-parser-output .refbegin-hanging-indents>ul>li,.mw-parser-output .refbegin-hanging-indents>dl>dd{margin-left:0;padding-left:3.2em;text-indent:-3.2em;list-style:none}.mw-parser-output .refbegin-100{font-size:100%}
0–4
5–6
7–9
10–15
16–25
26–33
34–70
71–121
122–162
163–235
As of 2012, pancreatic cancer resulted in 330,000 deaths globally,[6] up from 310,000 in 2010 and 200,000 in 1990.[99] In 2014, an estimated 46,000 people in the US are expected to be diagnosed with pancreatic cancer and 40,000 to die of it.[2] Although it accounts for only 2.5% of new cases, pancreatic cancer is responsible for 6% of cancer deaths each year.[100] It is the seventh highest cause of death from cancer worldwide.[6]
Globally pancreatic cancer is the 11th most common cancer in women and the 12th most common in men.[6] The majority of recorded cases occur in developed countries.[6] People from the United States have an average lifetime risk of about 1 in 67 (or 1.5%) of developing the disease,[101] slightly higher than the figure for the UK.[102] The disease is more common in men than women,[6][2] though the difference in rates has narrowed over recent decades, probably reflecting earlier increases in female smoking. In the United States the risk for African Americans is over 50% greater than for whites, but the rates in Africa and East Asia are much lower than those in North America or Europe. The United States, Central and eastern Europe, and Argentina and Uruguay all have high rates.[6]
Pancreatic cancer is the 10th most common cancer in the UK (around 8,800 people were diagnosed with the disease in 2011), and it is the 5th most common cause of cancer death (around 8,700 people died in 2012).[103]
PanNETs
The annual incidence of clinically recognized PanNETs is low (about 5 per one million person-years) and is dominated by the non-functioning types.[25] Somewhere between 45% and 90% of PanNETs are thought to be of the non-functioning types.[21][28] Studies of autopsies have uncovered small PanNETs rather frequently, suggesting that the prevalence of tumors that remain inert and asymptomatic may be relatively high.[28] Overall PanNETs are thought to account for about 1 to 2% of all pancreatic tumors.[25] The definition and classification of PanNETs has changed over time, affecting what is known about their epidemiology and clinical relevance.[48]
History
The earliest recognition of pancreatic cancer has been attributed to the 18th-century Italian scientist Giovanni Battista Morgagni, the historical father of modern-day anatomic pathology, who claimed to have traced several cases of cancer in the pancreas. Many 18th and 19th-century physicians were skeptical about the existence of the disease, given the similar appearance of pancreatitis. Some case reports were published in the 1820s and 1830s, and a genuine histopathologic diagnosis was eventually recorded by the American clinician Jacob Mendes Da Costa, who also doubted the reliability of Morgagni's interpretations. By the start of the 20th century, cancer of the head of the pancreas had become a well-established diagnosis.[104]
Regarding the recognition of PanNETs, the possibility of cancer of the islet cells was initially suggested in 1888. The first case of hyperinsulinism due to a tumor of this type was reported in 1927. Recognition of a non-insulin-secreting type of PanNET is generally ascribed to the American surgeons, R. M. Zollinger and E. H. Ellison, who gave their names to Zollinger–Ellison syndrome, after postulating the existence of a gastrin-secreting pancreatic tumor in a report of two cases of unusually severe peptic ulcers published in 1955.[104] In 2010, the WHO recommended that PanNETs be referred to as "neuroendocrine" rather than "endocrine" tumors.[27]
The first reported partial pancreaticoduodenectomy was performed by the Italian surgeon Alessandro Codivilla in 1898, but the patient only survived 18 days before succumbing to complications. Early operations were compromised partly because of mistaken beliefs that people would die if their duodenum were removed, and also, at first, if the flow of pancreatic juices stopped. Later it was thought, also mistakenly, that the pancreatic duct could simply be tied up without serious adverse effects; in fact, it will very often leak later on. In 1907–08, after some more unsuccessful operations by other surgeons, experimental procedures were tried on corpses by French surgeons.[105]
In 1912 the German surgeon Walther Kausch was the first to remove large parts of the duodenum and pancreas together (en bloc). This was in Breslau, now Wrocław in Poland. In 1918 it was demonstrated in operations on dogs that total removal of the duodenum is compatible with life, but this was not reported in human surgery until 1935, when the American surgeon Allen Oldfather Whipple published the results of a series of three operations at Columbia Presbyterian Hospital in New York. Only one of the patients had the duodenum totally removed, but he survived for two years before dying of metastasis to the liver. The first operation was unplanned, as cancer was only discovered in the operating theater. Whipple's success showed the way for the future, but the operation remained a difficult and dangerous one until recent decades. He published several refinements to his procedure, including the first total removal of the duodenum in 1940, but he only performed a total of 37 operations.[105]
The discovery in the late 1930s that vitamin K prevented bleeding with jaundice, and the development of blood transfusion as an everyday process, both improved post-operative survival,[105] but about 25% of people never left hospital alive as late as the 1970s.[106] In the 1970s a group of American surgeons wrote urging that the procedure was too dangerous and should be abandoned. Since then outcomes in larger centers have improved considerably, and mortality from the operation is often less than 4%.[23] In 2006 a report was published of a series of 1,000 consecutive pancreaticoduodenectomies performed by a single surgeon from Johns Hopkins Hospital between 1969 and 2003. The rate of these operations had increased steadily over this period, with only three of them before 1980, and the median operating time reduced from 8.8 hours in the 1970s to 5.5 hours in the 2000s, and mortality within 30 days or in hospital was only 1%.[105][106] Another series of 2,050 operations at the Massachusetts General Hospital between 1941 and 2011 showed a similar picture of improvement.[107]
Small precancerous neoplasms for many pancreatic cancers are being detected at greatly increased rates by modern medical imaging. One type, the intraductal papillary mucinous neoplasm (IPMN) was first described by Japanese researchers in 1982. It was noted in 2010 that: "For the next decade, little attention was paid to this report; however, over the subsequent 15 years, there has been a virtual explosion in the recognition of this tumor."[55]
Research directions
Worldwide efforts on many levels are underway to understand pancreatic cancer, but progress has been slow, particularly into understanding the disease's causes.[108] There are several fundamental unanswered questions.[109][110] The nature of the changes that lead to the disease are being intensely investigated, such as the roles played by genes such as KRAS and p53.[39][111][112] A key question is the timing of events as the disease develops and progresses – particularly the role of diabetes,[113] and how and when the disease spreads.[114]
Research on early detection is ongoing.[59][60] For instance, the European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC) trial is aiming to determine whether regular screening is appropriate for people with a family history of the disease, or who have hereditary pancreatitis.[115] The knowledge that new onset of diabetes can be an early sign of the disease could facilitate timely diagnosis and prevention if a workable screening strategy can be developed.[113][116][117]
Another area of interest is in assessing whether keyhole surgery (laparoscopy) would be better than Whipple's procedure in treating the disease surgically, particularly in terms of recovery time.[118]Irreversible electroporation is a relatively novel ablation technique that has shown promise in downstaging and prolonging survival in persons with locally advanced disease. It is especially suitable for treatment of tumors that are in proximity to peri-pancreatic vessels without risk of vascular trauma.[119][120] The limited success of outcomes after surgery has led to a number of trials that were running in 2014 to test outcomes using chemotherapy or radiochemotherapy before surgery. This had previously not been found to be helpful, but is being trialed again, using drug combinations which have emerged from the many trials of post-operative therapies, such as FOLFIRINOX.[2]
Efforts are underway to develop new drugs.[39][121] Some of these involve targeted therapies against the cancer cells' molecular mechanisms.[122][123][124] Others aim to target the highly resistant cancer stem cells.[72][125] Still others aim to affect the non-neoplastic stroma and microenvironment of the tumor, which is known to influence cell proliferation and metastasis.[124][125][126] A further approach involves the use of immunotherapy, such as oncolytic viruses.[127]
See also
- Gastrointestinal cancer
Pancreatic Cancer Action Network (organization in the US)
Pancreatic Cancer Action (organization in the UK)
Lustgarten Foundation for Pancreatic Cancer Research (organization in the US)- List of people diagnosed with pancreatic cancer
References
^ abcdefg "Pancreatic Cancer Treatment (PDQ®) Patient Version". National Cancer Institute. National Institutes of Health. 17 April 2014. Archived from the original on 5 July 2014. Retrieved 8 June 2014..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcdefghijklmnopqrstuvwxyzaaabacadaeafag Ryan DP, Hong TS, Bardeesy N (September 2014). "Pancreatic adenocarcinoma" (PDF). N. Engl. J. Med. 371 (11): 1039–49. doi:10.1056/NEJMra1404198. PMID 25207767. Archived from the original (PDF) on 26 December 2014.
^ abcdefghijklmnopqrstuvwxyzaaabacadaeafag Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH (September 2013). "Recent progress in pancreatic cancer". CA: A Cancer Journal for Clinicians. 63 (5): 318–48. doi:10.3322/caac.21190. PMC 3769458. PMID 23856911.
^ abcdefghij Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (August 2011). "Pancreatic cancer" (PDF). Lancet. 378 (9791): 607–20. doi:10.1016/S0140-6736(10)62307-0. PMC 3062508. PMID 21620466. Archived from the original (PDF) on 12 January 2015.
^ ab "Can pancreatic cancer be prevented?". American Cancer Society. 11 June 2014. Archived from the original on 13 November 2014. Retrieved 13 November 2014.
^ abcdefghijklmnopqrstu World Cancer Report 2014. World Health Organization. 2014. Chapter 5.7. ISBN 978-92-832-0429-9.
^ ab "Cancer Facts & Figures 2010" (PDF). American Cancer Society. 2010. Archived from the original (PDF) on 14 January 2015. Retrieved 5 December 2014. See p. 4 for incidence estimates, and p. 19 for survival percentages.
^ GBD 2015 Disease and Injury Incidence and Prevalence, Collaborators. (8 October 2016). "Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1545–1602. doi:10.1016/S0140-6736(16)31678-6. PMC 5055577. PMID 27733282.
^ ab GBD 2015 Mortality and Causes of Death, Collaborators. (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
^ "What is Cancer? Defining Cancer". National Cancer Institute, National Institutes of Health. 7 March 2014. Archived from the original on 25 June 2014. Retrieved 5 December 2014.
^ abcdefghijklmnopqrstuvw Bond-Smith G, Banga N, Hammond TM, Imber CJ (2012). "Pancreatic adenocarcinoma" (PDF). BMJ (Clinical Research Ed.). 344: e2476. doi:10.1136/bmj.e2476. PMID 22592847. Archived from the original (PDF) on 9 January 2015.
^ Bussom S, Saif MW (5 March 2010). "Methods and rationale for the early detection of pancreatic cancer. Highlights from the "2010 ASCO Gastrointestinal Cancers Symposium". Orlando, FL, USA. January 22–24, 2010". Journal of the Pancreas. 11 (2): 128–30. PMID 20208319. Archived from the original on 8 December 2014.
^ "Draft Recommendation Statement: Pancreatic Cancer: Screening - US Preventive Services Task Force". www.uspreventiveservicestaskforce.org. Retrieved 11 February 2019.
^ Shahrokni A, Saif MW (10 July 2013). "Metastatic pancreatic cancer: the dilemma of quality vs. quantity of life". Journal of the Pancreas. 14 (4): 391–4. doi:10.6092/1590-8577/1663. PMID 23846935.
^ Bardou M, Le Ray I (December 2013). "Treatment of pancreatic cancer: A narrative review of cost-effectiveness studies". Best Practice & Research. Clinical Gastroenterology. 27 (6): 881–92. doi:10.1016/j.bpg.2013.09.006. PMID 24182608.
^ Pancreatic Cancer Research Fund, 2015 Archived 27 November 2015 at the Wayback Machine
^ Hariharan D, Saied A, Kocher HM (2008). "Analysis of mortality rates for pancreatic cancer across the world". HPB. 10 (1): 58–62. doi:10.1080/13651820701883148. PMC 2504856. PMID 18695761.
^ "Lifetime Risk of Developing or Dying From Cancer". American Cancer Society. 1 October 2014. Archived from the original on 1 December 2014. Retrieved 1 December 2014.. The top three vary by sex, including breast cancer for women and prostate cancer for men.
^ ab "Pancreatic Cancer Treatment (PDQ®) Health Professional Version". National Cancer Institute. National Institutes of Health. 21 February 2014. Archived from the original on 22 October 2014. Retrieved 24 November 2014. "The highest cure rate occurs if the tumor is truly localized to the pancreas; however, this stage of disease accounts for less than 20% of cases. In cases with localized disease and small cancers (<2 cm) with no lymph node metastases and no extension beyond the capsule of the pancreas, complete surgical resection is still associated with a low actuarial five-year survival rate of 18% to 24%."
^ Harris, RE (2013). "Epidemiology of pancreatic cancer". Epidemiology of Chronic Disease. Jones & Bartlett. pp. 181–190. ISBN 978-0-7637-8047-0. Archived from the original on 24 June 2016.
^ abcdefg Öberg K, Knigge U, Kwekkeboom D, Perren A (October 2012). "Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 23 Suppl 7: vii124–30. doi:10.1093/annonc/mds295. PMID 22997445. Archived from the original on 11 October 2013. (Table 5 outlines the proposed TNM staging system for PanNETs.)
^ Handbook of Pancreatic Cancer. New York: Springer. 2009. p. 288. ISBN 978-0-387-77497-8. Archived from the original on 10 September 2017. Retrieved 12 June 2016.
^ ab Govindan R (2011). DeVita, Hellman, and Rosenberg's Cancer: Cancer: Principles & Practice of Oncology (9th ed.). Lippincott Williams & Wilkins. Chapter 35: Cancer of the Pancreas: Surgical Management. ISBN 978-1-4511-0545-2. Online edition, with updates to 2014
^ abc Tobias JS, Hochhauser D (2014). Cancer and its Management (7th ed.). p. 297. ISBN 978-1-118-46871-5.
^ abc "Types of Pancreas Tumors". The Sol Goldman Pancreas Cancer Research Center. Johns Hopkins Medicine. 2012. Archived from the original on 8 October 2014. Retrieved 18 November 2014.
^ Farrell JJ, Fernández-del Castillo C (June 2013). "Pancreatic cystic neoplasms: management and unanswered questions". Gastroenterology. 144 (6): 1303–15. doi:10.1053/j.gastro.2013.01.073. PMID 23622140.
^ abc The PanNET denomination is in line with WHO guidelines for the classification of tumors of the digestive system "Archived copy". Archived from the original on 9 September 2017. Retrieved 7 September 2017.CS1 maint: Archived copy as title (link) published in 2010. Historically, PanNETs have also been referred to by a variety of terms, and are still commonly called "pancreatic endocrine tumors". See: Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (August 2010). "The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems" (PDF). Pancreas. 39 (6): 707–12. doi:10.1097/MPA.0b013e3181ec124e. PMID 20664470.
^ abcdefgh Burns WR, Edil BH (March 2012). "Neuroendocrine pancreatic tumors: guidelines for management and update". Current Treatment Options in Oncology. 13 (1): 24–34. doi:10.1007/s11864-011-0172-2. PMID 22198808.
^ The Medical Subject Headings indexing system refers to "islet cell carcinoma", which is subdivided into gastrinoma, glucagonoma, somatostatinoma and VIPoma. See: 2014 MeSH tree at "Pancreatic Neoplasms [C04.588.322.475]" Archived 19 March 2016 at the Wayback Machine 16 October 2014
^ ab "Islet Cell Tumors of the Pancreas / Endocrine Neoplasms of the Pancreas". The Sol Goldman Pancreas Cancer Research Center. Johns Hopkins Medicine. 2012. Archived from the original on 5 January 2015. Retrieved 5 January 2015.
^ abcdefg De La Cruz MS, Young AP, Ruffin MT (April 2014). "Diagnosis and management of pancreatic cancer". Am Fam Physician. 89 (8): 626–32. PMID 24784121.
^ ab Alberts, SR; Goldberg, RM (2009). "Chapter 9: Gastrointestinal tract cancers". In Casciato, DA; Territo, MC. Manual of clinical oncology. Lippincott Williams & Wilkins. pp. 188–236. ISBN 978-0-7817-6884-9.
^ Pannala R, Basu A, Petersen GM, Chari ST (January 2009). "New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer". The Lancet. Oncology. 10 (1): 88–95. doi:10.1016/S1470-2045(08)70337-1. PMC 2795483. PMID 19111249.
^ "Chapter 15; Pancreas". Manual for Staging of Cancer (PDF) (2nd ed.). American Joint Committee on Cancer. pp. 95–8. Archived (PDF) from the original on 29 November 2014. See page 95 for citation regarding "... lesser degree of involvement of bones and brain and other anatomical sites."
^ Sperti C, Moletta L, Patanè G (15 October 2014). "Metastatic tumors to the pancreas: The role of surgery". World Journal of Gastrointestinal Oncology. 6 (10): 381–92. doi:10.4251/wjgo.v6.i10.381. PMC 4197429. PMID 25320654.
^ "Causes of pancreatic cancer". NHS Choices. National Health Service, England. 7 October 2014. Archived from the original on 6 November 2014. Retrieved 5 December 2014.
^ Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, Li D, Risch HA, Olson SH, Gallinger S, Miller AB, Bueno-de-Mesquita HB, Talamini R, Polesel J, Ghadirian P, Baghurst PA, Zatonski W, Fontham E, Bamlet WR, Holly EA, Bertuccio P, Gao YT, Hassan M, Yu H, Kurtz RC, Cotterchio M, Su J, Maisonneuve P, Duell EJ, Boffetta P, La Vecchia C (July 2012). "Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4)". Annals of Oncology. 23 (7): 1880–8. doi:10.1093/annonc/mdr541. PMC 3387822. PMID 22104574.
^ Peters, ML; Tseng, JF; Miksad, RA (31 March 2016). "Genetic Testing in Pancreatic Ductal Adenocarcinoma: Implications for Prevention and Treatment". Clinical Therapeutics. 38 (7): 1622–35. doi:10.1016/j.clinthera.2016.03.006. PMID 27041411.
^ abcd Reznik R, Hendifar AE, Tuli R (2014). "Genetic determinants and potential therapeutic targets for pancreatic adenocarcinoma". Front Physiol. 5: 87. doi:10.3389/fphys.2014.00087. PMC 3939680. PMID 24624093.
^ Greenhalf W, Grocock C, Harcus M, Neoptolemos J (2009). "Screening of high-risk families for pancreatic cancer". Pancreatology. 9 (3): 215–22. doi:10.1159/000210262. PMID 19349734. Archived from the original on 10 September 2017.
^ ab "Cancer Facts and Figures 2014" (PDF). American Cancer Society. Archived (PDF) from the original on 18 December 2014. Retrieved 5 January 2015., p. 19, "Though evidence is still accumulating, consumption of red or processed meat, or meat cooked at very high temperatures, may slightly increase risk."
^ Larsson SC, Wolk A (January 2012). "Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies". Br J Cancer. 106 (3): 603–7. doi:10.1038/bjc.2011.585. PMC 3273353. PMID 22240790. Archived from the original on 15 January 2012.
^ ab Pericleous M, Rossi RE, Mandair D, Whyand T, Caplin ME (January 2014). "Nutrition and pancreatic cancer". Anticancer Research. 34 (1): 9–21. PMID 24403441.
^ Delpu Y, Hanoun N, Lulka H, Sicard F, Selves J, Buscail L, Torrisani J, Cordelier P (2011). "Genetic and epigenetic alterations in pancreatic carcinogenesis". Curr Genomics. 12 (1): 15–24. doi:10.2174/138920211794520132. PMC 3129039. PMID 21886451.
^ Rooney, SL; Shi, J (October 2016). "Intraductal Tubulopapillary Neoplasm of the Pancreas: An Update From a Pathologist's Perspective". Archives of Pathology & Laboratory Medicine. 140 (10): 1068–73. doi:10.5858/arpa.2016-0207-RA. PMID 27684978.
^ ab "The human pathology proteome in pancreatic cancer – The Human Protein Atlas". www.proteinatlas.org. Retrieved 28 September 2017.
^ ab Uhlen, Mathias; Zhang, Cheng; Lee, Sunjae; Sjöstedt, Evelina; Fagerberg, Linn; Bidkhori, Gholamreza; Benfeitas, Rui; Arif, Muhammad; Liu, Zhengtao (18 August 2017). "A pathology atlas of the human cancer transcriptome". Science. 357 (6352): eaan2507. doi:10.1126/science.aan2507. ISSN 0036-8075. PMID 28818916.
^ ab Lewis MA, Yao JC (February 2014). "Molecular pathology and genetics of gastrointestinal neuroendocrine tumours". Current Opinion in Endocrinology, Diabetes and Obesity. 21 (1): 22–7. doi:10.1097/MED.0000000000000033. PMID 24310147.
^ Thakker RV, Newey PJ, Walls GV, Bilezikian J, Dralle H, Ebeling PR, Melmed S, Sakurai A, Tonelli F, Brandi ML (September 2012). "Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1)" (PDF). The Journal of Clinical Endocrinology and Metabolism. 97 (9): 2990–3011. doi:10.1210/jc.2012-1230. PMID 22723327. Archived (PDF) from the original on 17 February 2015.
^ Fitzgerald JE, White MJ, Lobo DN (April 2009). "Courvoisier's gallbladder: law or sign?" (PDF). World Journal of Surgery. 33 (4): 886–91. doi:10.1007/s00268-008-9908-y. PMID 19190960. Archived from the original on 5 January 2015.CS1 maint: BOT: original-url status unknown (link)
^ Cyrus Piraka; James M. Scheiman (2011). "New Diagnostic Imaging Modalities for Pancreatic Disease". Curr Opin Gastroenterol. 27 (5). Archived from the original on 25 November 2013.
^ abcd Seufferlein T, Bachet JB, Van Cutsem E, Rougier P (October 2012). "Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 23 Suppl 7: vii33–40. doi:10.1093/annonc/mds224. PMID 22997452.
^ Cascinu S, Falconi M, Valentini V, Jelic S (May 2010). "Pancreatic cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of Oncology. 21 Suppl 5: v55–8. doi:10.1093/annonc/mdq165. PMID 20555103. Archived from the original on 17 August 2011.
^ abc "Staging of pancreatic cancer". American Cancer Society. 11 June 2014. Retrieved 29 September 2014.
^ ab Zyromski, Nicholas J.; Nakeeb, Attila; Lillemoe, Keith D. (2010). Silberman, Howard; Silberman, Allan W., eds. Principles and practice of surgical oncology : multidisciplinary approach to difficult problems (online ed.). Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins. Chapter 35. ISBN 978-0-7817-6546-6. Archived from the original on 6 February 2015. Retrieved 3 November 2014.CS1 maint: BOT: original-url status unknown (link)
^ abcd "Neuroendocrine tumors, NCCN Guidelines Version 1.2015" (PDF). NCCN Guidelines. National Comprehensive Cancer Network, Inc. 11 November 2014. Retrieved 25 December 2014.
^ National Cancer Institute. Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment (PDQ®) Incidence and Mortality "Archived copy". Archived from the original on 4 January 2015. Retrieved 29 December 2014.CS1 maint: Archived copy as title (link)
^ "Diet and activity factors that affect risks for certain cancers: Pancreatic cancer section". American Cancer Society. 20 August 2012. Archived from the original on 4 November 2014. Retrieved 4 November 2014.
^ ab He XY, Yuan YZ (August 2014). "Advances in pancreatic cancer research: moving towards early detection". World J. Gastroenterol. 20 (32): 11241–8. doi:10.3748/wjg.v20.i32.11241. PMC 4145762. PMID 25170208.
^ abc Okano K, Suzuki Y (August 2014). "Strategies for early detection of resectable pancreatic cancer". World J. Gastroenterol. 20 (32): 11230–40. doi:10.3748/wjg.v20.i32.11230. PMC 4145761. PMID 25170207.
^ Stoita A, Penman ID, Williams DB (May 2011). "Review of screening for pancreatic cancer in high risk individuals". World J. Gastroenterol. 17 (19): 2365–71. doi:10.3748/wjg.v17.i19.2365. PMC 3103788. PMID 21633635.
^ Gurusamy KS, Kumar S, Davidson BR, Fusai G (2014). "Cochrane Database of Systematic Reviews". The Cochrane Database of Systematic Reviews. 2 (2): CD010244. doi:10.1002/14651858.CD010244.pub2. PMID 24578248.
^ ab Mollberg N, Rahbari NN, Koch M, Hartwig W, Hoeger Y, Büchler MW, Weitz J (2011). "Arterial resection during pancreatectomy for pancreatic cancer: A systematic review and meta-analysis". Annals of Surgery. 254 (6): 882–93. doi:10.1097/SLA.0b013e31823ac299. PMID 22064622.
^ abc "Pancreatic adenocarcinoma. NCCN Guidelines Version 1.2015" (PDF). NCCN Guidelines. National Comprehensive Cancer Network, Inc. 4 December 2014. Retrieved 26 December 2014.
^ Alamo JM, Marín LM, Suarez G, Bernal C, Serrano J, Barrera L, Gómez MA, Muntané J, Padillo FJ (2014). "Improving outcomes in pancreatic cancer: key points in perioperative management". World J. Gastroenterol. 20 (39): 14237–45. doi:10.3748/wjg.v20.i39.14237. PMC 4202352. PMID 25339810.
^ Lopez NE, Prendergast C, Lowy AM (2014). "Borderline resectable pancreatic cancer: definitions and management". World J. Gastroenterol. 20 (31): 10740–51. doi:10.3748/wjg.v20.i31.10740. PMC 4138454. PMID 25152577.
^ Polistina F, Di Natale G, Bonciarelli G, Ambrosino G, Frego M (2014). "Neoadjuvant strategies for pancreatic cancer". World J. Gastroenterol. 20 (28): 9374–83. doi:10.3748/wjg.v20.i28.9374 (inactive 2018-09-23). PMC 4110569. PMID 25071332.
^ Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J (2010). "Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages". PLoS Med. 7 (4): e1000267. doi:10.1371/journal.pmed.1000267. PMC 2857873. PMID 20422030.
^ Christians KK, Evans DB (2014). "Additional Support for Neoadjuvant Therapy in the Management of Pancreatic Cancer". Ann. Surg. Oncol. 22 (6): 1755–8. doi:10.1245/s10434-014-4307-0. PMID 25519932.
^ Tsvetkova EV, Asmis TR (2014). "Role of neoadjuvant therapy in the management of pancreatic cancer: is the era of biomarker-directed therapy here?". Curr Oncol. 21 (4): e650–7. doi:10.3747/co.21.2006. PMC 4117630. PMID 25089113.
^ Zhan HX, Xu JW, Wu D, Zhang TP, Hu SY (2015). "Pancreatic cancer stem cells: New insight into a stubborn disease". Cancer Lett. 357 (2): 429–37. doi:10.1016/j.canlet.2014.12.004. PMID 25499079.
^ ab Tanase CP, Neagu AI, Necula LG, Mambet C, Enciu AM, Calenic B, Cruceru ML, Albulescu R (2014). "Cancer stem cells: Involvement in pancreatic cancer pathogenesis and perspectives on cancer therapeutics". World Journal of Gastroenterology. 20 (31): 10790–801. doi:10.3748/wjg.v20.i31.10790. PMC 4138459. PMID 25152582.
^ Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR (2016). "Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer". Cochrane Database Syst Rev. 7: CD009323. doi:10.1002/14651858.CD009323.pub3. PMID 27383694.
^ Heinemann V, Haas M, Boeck S (October 2013). "Neoadjuvant treatment of borderline resectable and non-resectable pancreatic cancer". Annals of Oncology. 24 (10): 2484–92. doi:10.1093/annonc/mdt239. PMID 23852311.
^ abc Thota R, Pauff JM, Berlin JD (January 2014). "Treatment of metastatic pancreatic adenocarcinoma: a review". Oncology (Williston Park, N.Y.). 28 (1): 70–4. PMID 24683721.
^ Ryan, DP (8 July 2014). "Chemotherapy for advanced exocrine pancreatic cancer: Topic 2475, Version 46.0" (subscription required). UpToDate. Wolters Kluwer Health. Archived from the original on 8 December 2014. Retrieved 18 November 2014.
^ "Cancer Drug Information: FDA Approval for Erlotinib Hydrochloride". National Cancer Institute. National Institutes of Health. 3 July 2013. Archived from the original on 29 November 2014. Retrieved 5 December 2014.
^ Borazanci E, Von Hoff DD; Von Hoff, DD (September 2014). "Nab-paclitaxel and gemcitabine for the treatment of people with metastatic pancreatic cancer". Expert Rev Gastroenterol Hepatol. 8 (7): 739–47. doi:10.1586/17474124.2014.925799. PMID 24882381.
^ Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O'Connor JM, Salazar R, Taal BG, Vullierme MP, O'Toole D (2012). "ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: Well-differentiated pancreatic non-functioning tumors". Neuroendocrinology. 95 (2): 120–34. doi:10.1159/000335587. PMID 22261872.
^ Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R (2012). "ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes". Neuroendocrinology. 95 (2): 98–119. doi:10.1159/000335591. PMC 3701449. PMID 22261919.
^ Pavel M, Baudin E, Couvelard A, Krenning E, Öberg K, Steinmüller T, Anlauf M, Wiedenmann B, Salazar R (2012). "ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary". Neuroendocrinology. 95 (2): 157–76. doi:10.1159/000335597. PMID 22262022.
^ Rossi RE, Massironi S, Conte D, Peracchi M (2014). "Therapy for metastatic pancreatic neuroendocrine tumors". Annals of Translational Medicine. 2 (1): 8. doi:10.3978/j.issn.2305-5839.2013.03.01. PMC 4200651. PMID 25332984.
^ Nick Mulcahy (17 December 2014). "FDA Approves Lanreotide for Neuroendocrine Tumors". Medscape Medical News. WebMD LLC. Archived from the original on 18 January 2015. Retrieved 25 December 2014.
^ Everolimus Approved for Pancreatic Neuroendocrine Tumors Archived 16 July 2016 at the Wayback Machine The ASCO Post. 15 May 2011, Volume 2, Issue 8
^ National Cancer Institute. Cancer Drug Information. FDA Approval for Sunitinib Malate Archived 5 January 2015 at the Wayback Machine. Pancreatic Neuroendocrine Tumors
^ Tejani MA, Saif MW (2014). "Pancreatic neuroendocrine tumors: Does chemotherapy work?". Journal of the Pancreas. 15 (2): 132–4. doi:10.6092/1590-8577/2301. PMID 24618436.
^ Text is available electronically (but may require free registration) See: Benson AB, Myerson RJ, Sasson AR. Pancreatic, neuroendocrine GI, and adrenal cancers. Cancer Management: A Multidisciplinary Approach 13th edition 2010. ISBN 978-0-615-41824-7. Archived from the original on 15 May 2011.
^ Gulenchyn KY, Yao X, Asa SL, Singh S, Law C (2012). "Radionuclide therapy in neuroendocrine tumours: A systematic review". Clinical Oncology. 24 (4): 294–308. doi:10.1016/j.clon.2011.12.003. PMID 22221516.
^ Vinik AI (2014). "Advances in Diagnosis and Treatment of Pancreatic Neuroendocrine Tumors (PNETS)". Endocrine Practice. 20 (11): 1–23. doi:10.4158/EP14373.RA. PMID 25297671.
^ Kwekkeboom DJ, de Herder WW, van Eijck CH, Kam BL, van Essen M, Teunissen JJ, Krenning EP (2010). "Peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors". Seminars in Nuclear Medicine. 40 (2): 78–88. doi:10.1053/j.semnuclmed.2009.10.004. PMID 20113677.
^ Bodei L, Cremonesi M, Kidd M, Grana CM, Severi S, Modlin IM, Paganelli G (2014). "Peptide receptor radionuclide therapy for advanced neuroendocrine tumors". Thoracic Surgery Clinics. 24 (3): 333–49. doi:10.1016/j.thorsurg.2014.04.005. PMID 25065935.
^ Castellano D, Grande E, Valle J, Capdevila J, Reidy-Lagunes D, O'Connor JM, Raymond E (2014). "Expert consensus for the management of advanced or metastatic pancreatic neuroendocrine and carcinoid tumors". Cancer Chemotherapy and Pharmacology. 75 (6): 1099–114. doi:10.1007/s00280-014-2642-2. PMID 25480314.
^ Singh S, Dey C, Kennecke H, Kocha W, Maroun J, Metrakos P, Mukhtar T, Pasieka J, Rayson D, Rowsell C, Sideris L, Wong R, Law C (2014). "Consensus Recommendations for the Diagnosis and Management of Pancreatic Neuroendocrine Tumors: Guidelines from a Canadian National Expert Group". Annals of Surgical Oncology. 22 (8): 2685–99. doi:10.1245/s10434-014-4145-0. PMID 25366583.
^ "Palliative or Supportive Care". American Cancer Society. 2014. Archived from the original on 21 August 2014. Retrieved 20 August 2014.
^ Buanes TA (14 August 2014). "Pancreatic cancer-improved care achievable". World Journal of Gastroenterology. 20 (30): 10405–18. doi:10.3748/wjg.v20.i30.10405. PMC 4130847. PMID 25132756.
^ "If treatment for pancreatic cancer stops working". American Cancer Society. 11 June 2014. Archived from the original on 22 October 2014. Retrieved 20 August 2014.
^ Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA (2011). Arcidiacono PG, ed. "Celiac plexus block for pancreatic cancer pain in adults". Cochrane Database Syst Rev (3): CD007519. doi:10.1002/14651858.CD007519.pub2. PMID 21412903.
^ "Cancer Facts and Figures 2014" (PDF). American Cancer Society. Archived (PDF) from the original on 18 December 2014. Retrieved 5 January 2015., Table, p. 18, rates adjusted for normal life expectancy
^ Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, et al. (December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
^ Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007). "Cancer statistics, 2007". CA. 57 (1): 43–66. doi:10.3322/canjclin.57.1.43. PMID 17237035.
^ "What are the key statistics about pancreatic cancer?". American Cancer Society. 11 June 2014. Archived from the original on 11 November 2014. Retrieved 11 November 2014.
^ "Pancreatic cancer statistics". Cancer Research UK. Archived from the original on 18 December 2014. Retrieved 18 December 2014.; "In 2010, in the UK, the lifetime risk of developing pancreatic cancer is 1 in 73 for men and 1 in 74 for women", noting "The lifetime risk ... has been calculated ... using the 'Current Probability' method; this is a different method used from most other cancer sites since the possibility of having more than one diagnosis of pancreatic cancer over the course of their lifetime is very low"
^ "Pancreatic cancer statistics". Cancer Research UK. Archived from the original on 6 October 2014. Retrieved 28 October 2014.
^ ab Busnardo AC, DiDio LJ, Tidrick RT, Thomford NR (1983). "History of the pancreas" (PDF). American Journal of Surgery. 146 (5): 539–50. doi:10.1016/0002-9610(83)90286-6. PMID 6356946.
^ abcd Are C, Dhir M, Ravipati L (June 2011). "History of pancreaticoduodenectomy: early misconceptions, initial milestones and the pioneers". HPB. 13 (6): 377–84. doi:10.1111/j.1477-2574.2011.00305.x. PMC 3103093. PMID 21609369.
^ ab Cameron JL, Riall TS, Coleman J, Belcher KA (July 2006). "One thousand consecutive pancreaticoduodenectomies". Annals of Surgery. 244 (1): 10–5. doi:10.1097/01.sla.0000217673.04165.ea. PMC 1570590. PMID 16794383.
^ Fernández-del Castillo C, Morales-Oyarvide V, McGrath D, Wargo JA, Ferrone CR, Thayer SP, Lillemoe KD, Warshaw AL (September 2012). "Evolution of the Whipple procedure at the Massachusetts General Hospital". Surgery. 152 (3 Suppl 1): S56–63. doi:10.1016/j.surg.2012.05.022. PMC 3806095. PMID 22770961.
^ Wolpin BM, Stampfer MJ (July 2009). "Defining determinants of pancreatic cancer risk: are we making progress?". J. Natl. Cancer Inst. 101 (14): 972–3. doi:10.1093/jnci/djp182. PMID 19561317.
^ "What's new in pancreatic cancer research and treatment?". American Cancer Society. 11 June 2014. Archived from the original on 10 July 2014. Retrieved 17 July 2014.
^ "Pancreatic cancer research". Cancer Research UK. Archived from the original on 18 February 2014. Retrieved 17 July 2014.
^ "Australian Pancreatic Genome Initiative". Garvan Institute. Archived from the original on 26 July 2014. Retrieved 17 July 2014.
^ Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, et al. (November 2012). "Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes". Nature. 491 (7424): 399–405. Bibcode:2012Natur.491..399.. doi:10.1038/nature11547. PMC 3530898. PMID 23103869.
^ ab Pannala R, Basu A, Petersen GM, Chari ST (January 2009). "New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer". Lancet Oncol. 10 (1): 88–95. doi:10.1016/S1470-2045(08)70337-1. PMC 2795483. PMID 19111249.
^ Graham JS, Jamieson NB, Rulach R, Grimmond SM, Chang DK, Biankin AV (November 2014). "Pancreatic cancer genomics: where can the science take us?". Clin. Genet. 88 (3): 213–9. doi:10.1111/cge.12536. PMID 25388820.
^ "About EUROPAC". European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC). University of Liverpool. Archived from the original on 26 July 2014. Retrieved 17 July 2014.
^ Zhang C, Yang G, Ling Y, Chen G, Zhou T (December 2014). "The early diagnosis of pancreatic cancer and diabetes: what's the relationship?". Journal of Gastrointestinal Oncology. 5 (6): 481–8. doi:10.3978/j.issn.2078-6891.2014.055. PMC 4226830. PMID 25436129.
^ Bruenderman EH, Martin RC (13 October 2014). "High-risk population in sporadic pancreatic adenocarcinoma: guidelines for screening". The Journal of Surgical Research. 194 (1): 212–219. doi:10.1016/j.jss.2014.06.046. PMC 4559279. PMID 25479908.
^ Subar D, Gobardhan PD, Gayet B (2014). "Laparoscopic pancreatic surgery". Best Practice & Research Clinical Gastroenterology. 28 (1): 123–32. doi:10.1016/j.bpg.2013.11.011. PMID 24485260.
^ Weiss MJ, Wolfgang CL (2013). "Irreversible electroporation: a novel pancreatic cancer therapy". Current Problems in Cancer. 37 (5): 262–5. doi:10.1016/j.currproblcancer.2013.10.002. PMID 24331180.
^ Moir J, White SA, French JJ, Littler P, Manas DM (December 2014). "Systematic review of irreversible electroporation in the treatment of advanced pancreatic cancer". European Journal of Surgical Oncology. 40 (12): 1598–1604. doi:10.1016/j.ejso.2014.08.480. PMID 25307210.
^ Al Haddad AH, Adrian TE (November 2014). "Challenges and future directions in therapeutics for pancreatic ductal adenocarcinoma". Expert Opin Investig Drugs. 23 (11): 1499–515. doi:10.1517/13543784.2014.933206. PMID 25078674.
^ Adiseshaiah, Pavan P.; Crist, Rachael M.; Hook, Sara S.; McNeil, Scott E. (2016). "Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer". Nature Reviews Clinical Oncology. 13 (12): 750–765. doi:10.1038/nrclinonc.2016.119. ISSN 1759-4774. PMID 27531700.
^ Kleger A, Perkhofer L, Seufferlein T (July 2014). "Smarter drugs emerging in pancreatic cancer therapy". Ann. Oncol. 25 (7): 1260–70. doi:10.1093/annonc/mdu013. PMID 24631947.
^ ab Tang SC, Chen YC (August 2014). "Novel therapeutic targets for pancreatic cancer". World Journal of Gastroenterology. 20 (31): 10825–44. doi:10.3748/wjg.v20.i31.10825. PMC 4138462. PMID 25152585. Archived from the original on 29 December 2014.
^ ab Schober M, Jesenofsky R, Faissner R, Weidenauer C, Hagmann W, Michl P, Heuchel RL, Haas SL, Löhr JM (2014). "Desmoplasia and chemoresistance in pancreatic cancer". Cancers (Basel). 6 (4): 2137–54. doi:10.3390/cancers6042137. PMC 4276960. PMID 25337831.
^ Rossi ML, Rehman AA, Gondi CS (2014). "Therapeutic options for the management of pancreatic cancer". World J. Gastroenterol. 20 (32): 11142–59. doi:10.3748/wjg.v20.i32.11142. PMC 4145755. PMID 25170201.
^ Fong Y, Ady J, Heffner J, Klein E (2014). "Oncolytic viral therapy for pancreatic cancer: current research and future directions". Oncolytic Virotherapy. 3: 35–46. doi:10.2147/OV.S53858. PMC 4918362. PMID 27512661.
External links
| Classification | D
|
|---|---|
| External resources |
|
| Wikimedia Commons has media related to Pancreatic cancer. |
Pancreatic cancer at Curlie




