Norethisterone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Alone: Aygestin, Camila, Heather, Micronor, Primolut N, others; With EE: Lo Loestrin, Loestrin, Microgestin, Modicon, Norinyl, Ortho-Novum, others; With E2: Activella, Activelle, Estalis, Kliogest, Necon, Novofem, Trisequens, others |
| Synonyms | NET; Norethindrone; NSC-9564; LG-202; Ethinylnortestosterone; Norpregneninolone; Anhydrohydroxy-norprogesterone; Ethinylestrenolone; 17α-Ethynyl-19-nortestosterone; 17α-Ethynylestra-4-en-17β-ol-3-one; 17α-Hydroxy-19-norpregn-4-en-20-yn-3-one |
AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a604034 |
| Routes of administration | By mouth, intramuscular injection (as NETE) |
| Drug class | Progestin; Progestogen |
| ATC code |
|
Pharmacokinetic data | |
| Bioavailability | 47–73% (mean 64%)[2][3] |
| Protein binding | 97%:[4] Albumin: 61%;[4] SHBG: 36%[4] |
| Metabolism | Mainly CYP3A4 (liver);[1] also 5α-/5β-reductase, 3α-/3β-HSD, and aromatase |
| Elimination half-life | 5.2–12.8 hours (mean 8.0 hours)[2] |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| ECHA InfoCard | 100.000.619 |
| Chemical and physical data | |
| Formula | C20H26O2 |
| Molar mass | 298.419 g/mol |
| 3D model (JSmol) |
|
| Melting point | 203 to 204 °C (397 to 399 °F) |
SMILES
| |
InChI
| |
.mw-parser-output .nobold{font-weight:normal} (verify) | |
Norethisterone, also known as norethindrone and sold under the brand names Aygestin and Primolut N among many others, is a progestin medication which is used in birth control pills, menopausal hormone therapy, and for the treatment of gynecological disorders.[4][5] The medication available in both low-dose and high-dose formulations and is used and formulated both alone and in combination with an estrogen.[5][6] It is taken by mouth.[4][5]
Side effects of norethisterone include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[7][8] Norethisterone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[4][5] It has weak androgenic and estrogenic activity, mostly at high dosages, and no other important hormonal activity.[4][9]
Norethisterone was discovered in 1951 and was one of the first progestins to be developed.[10][11][12] It was first introduced for medical use on its own in 1957 and was introduced in combination with an estrogen for use as a birth control pill in 1963.[12][13] It is sometimes referred to as a "first-generation" progestin.[14][15] Along with desogestrel, it is one of the only progestins that is widely available as a progestogen-only "mini pill" for birth control.[16][17] Norethisterone is marketed widely throughout the world.[18] It is available as a generic medication.[19]
In addition to norethisterone itself, a variety of prodrugs of norethisterone, such as norethisterone acetate (NETA) and norethisterone enanthate (NETE) among others, have been marketed and have similar effects and uses.[20][21][22] NETA is taken by mouth similarly to norethisterone, while NETE is given by injection into muscle.[9] Many derivatives of norethisterone, such as levonorgestrel and desogestrel, have also been developed and marketed.[4]
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 ul{display:none}
Contents
1 Medical uses
2 Contraindications
3 Side effects
3.1 Androgenic
3.2 Estrogenic
4 Overdose
5 Interactions
6 Pharmacology
6.1 Pharmacodynamics
6.1.1 Progestogenic activity
6.1.2 Androgenic activity
6.1.3 Estrogenic activity
6.1.4 Neurosteroid activity
6.1.5 Steroidogenesis inhibition
6.1.6 Other activities
6.2 Pharmacokinetics
6.2.1 Absorption
6.2.2 Distribution
6.2.3 Metabolism
6.2.4 Elimination
7 Chemistry
7.1 Derivatives
7.1.1 Non-17α-ethynylated
7.2 Synthesis
7.2.1 Synthesis 1
7.2.2 Synthesis 2
8 History
9 Society and culture
9.1 Generic names
9.2 Brand names
9.3 Availability
9.3.1 United States
10 Research
11 References
12 Further reading
Medical uses
Norethisterone is used as a hormonal contraceptive in combination with an estrogen – usually ethinylestradiol (EE) – in combined oral contraceptive pills and alone in progestogen-only pills. Norethisterone has also been shown to be effective in inhibiting leutinizing hormone and follicle-stimulating hormone secretion in men as well.[23] Without these hormones, mature sperm cannot survive in the testes. This means that norethisterone could also be an effective male hormonal contraceptive. Aside from its use as a contraceptive, norethisterone can be used to treat premenstrual syndrome, dysmenorrhea, menorrhagia, irregular menstruation, menopausal symptoms (in combination with estrogen), or to postpone a period. It is also commonly used to help prevent uterine hemorrhage in complicated non-surgical or pre-surgical gynecologic cases and in treating non responsive cyclical mastalgia.
Another medical use of norethisterone is to alleviate endometriosis related pain. In fact, 50% of patients who received medical or surgical treatment for endometriosis-related pelvic pain have benefited from progestin therapy. This could be due to the fact that norethisterone induces endometrial proliferation during secretory phase, which has been shown to alleviate endometrial pain complaints. Another way in which norethisterone may be acting to reduce endometrial pain is via inhibition of ovulation. Endometriosis pain and discomfort is worse during ovulation.[24]
Contraindications
High-dose (10 mg/day) norethisterone has been associated with hepatic veno-occlusive disease, and because of this adverse effect, norethisterone should not be given to patients undergoing allogeneic bone marrow transplantation, as it has been associated with substantially lower one-year survival post-transplantation.[25]:217[26]
Side effects
At contraceptive and hormone replacement dosages (0.35 to 1 mg/day), norethisterone has essentially progestogenic side effects only. In most clinical studies of norethisterone for contraception or menopausal hormone therapy, the drug has been combined with an estrogen, and for this reason, it is difficult to determine which of the side effects were caused by norethisterone and which of them were caused by estrogen in such research. However, NETE, an intramuscularly administered prodrug of norethisterone which is used as a long-acting contraceptive, is used without an estrogen, and hence can be employed as a surrogate for norethisterone in terms of understanding its effects and tolerability. In clinical studies, the most common side effect with NETE has been menstrual disturbances, including prolonged bleeding or spotting and amenorrhea.[25]:253 Other side effects have included periodic abdominal bloating and breast tenderness, both of which are thought to be due to water retention and can be relieved with diuretics.[25]:253 There has been no association with weight gain, and blood pressure, blood clotting, and glucose tolerance have all remained normal.[25]:253 However, a decrease in HDL cholesterol has been observed.[25]:253
At high dosages (5 to 60 mg/day), for instance those used in the treatment of gynecological disorders, norethisterone can cause hypogonadism due to its antigonadotropic effects and can have estrogenic and weak androgenic side effects.
Androgenic
Due to its weak androgenic activity, norethisterone can produce androgenic side effects such as acne, hirsutism, and voice changes of slight severity in some women at high dosages (e.g., 10 to 40 mg/day).[8] This is notably not the case with combined oral contraceptives that contain norethisterone and EE, however.[9] Such formulations contain low dosages of norethisterone (0.35 to 1 mg/day)[9] in combination with estrogen and are actually associated with improvement in acne symptoms.[27][28] In accordance, they are in fact approved by the FDA for the treatment of acne in women in the United States.[27][28] The improvement in acne symptoms is believed to be due to a 2- to 3-fold increase in sex hormone-binding globulin (SHBG) levels and a consequent decrease in free testosterone levels caused by EE, which results in an overall decrease in androgenic signaling in the body.[29]
The sebaceous glands are highly androgen-sensitive and their size and activity are potential markers of androgenic effect.[30] A high dosage of 20 mg/day norethisterone or NETA has been found to significantly stimulate the sebaceous glands, whereas lower dosages of 5 mg/day and 2.5 mg/day norethisterone and NETA, respectively, did not significantly stimulate sebum production and were consequently regarded as devoid of significant androgenicity.[30] Conversely, dosages of norethisterone of 0.5 to 3 mg/day have been found to dose-dependently decrease SHBG levels (and hence to suppress hepatic SHBG production), which is another highly sensitive marker of androgenicity.[31]
A large clinical study of high to very high oral dosages of norethisterone (10 to 40 mg/day) administered for prolonged periods of time (4 to 35 weeks) to prevent miscarriage in pregnant women found that 5.5% of the women experienced mild androgenic side effects such as mild voice changes (hoarseness), acne, and hirsutism and that 18.3% of female infants born to the mothers showed, in most cases only slight, virilization of the genitals.[8] Maternal androgenic symptoms occurred most often in women who received a dosage of norethisterone of 30 mg/day or more for a period of 15 weeks or longer.[8] In the female infants who experienced virilization of the genitals, the sole manifestation in 86.7% of the cases was varied but almost always slight enlargement of the clitoris.[8] In the remaining 13.3% of the affected cases, marked clitoral enlargement and partial fusion of the labioscrotal folds occurred.[8] The dosages used in these cases were 20 to 40 mg/day.[8]
In a letter to the editor on the topic of virilization caused by high dosages of NETA in women, a physician expressed that they had not observed the "slightest evidence of virilization" and that there had "certainly been no hirsutism nor any voice changes" in 55 women with advanced breast cancer that they had treated with 30 to 60 mg/day norethisterone for up to six months.[32]
High-dosage norethisterone has been used to suppress menstruation in women with severe intellectual disability who were incapable of handling their own menses.[33][34] A study of 118 nulliparous women treated with 5 mg/day norethisterone for a period of 2 to 30 months found that the drug was effective in producing amenorrhea in 86% of the women, with breakthrough bleeding occurring in the remaining 14%.[33] Side effects including weight gain, hirsutism, acne, headache, nausea, and vomiting all did not appear to increase in incidence and no "disturbing side effects" were noted in any of the women.[33][34] Another study of 5 mg/day norethisterone in 132 women also made no mention of androgenic side effects.[35] These findings suggest little to no risk of androgenic side effects with norethisterone at a dosage of 5 mg/day.[33][34] A study of 194 women treated with 5 to 15 mg/day NETA for a median duration of 13 months of therapy to suppress symptoms of endometriosis observed no side effects in 55.2% of patients, weight gain in 16.1%, acne in 9.9%, mood lability in 8.9%, hot flashes in 8.3%, and voice deepening in two women (1.0%).[36]
Estrogenic
Norethisterone is weakly estrogenic (via conversion into its metabolite EE), and for this reason, it has been found at high dosages to be associated with high rates of estrogenic side effects such as breast enlargement in women and gynecomastia in men, but also with improvement of menopausal symptoms in postmenopausal women.[37] It has been suggested that very high dosages (e.g., 40 mg/day, which are sometimes used in clinical practice for various indications) of NETA (and by extension norethisterone) may result in an increased risk of venous thromboembolism (VTE) analogously to high dosages (above 50 μg/day) of EE, and that even dosages of NETA of 10 to 20 mg, which correspond to EE dosages of approximately 20 to 30 μg/day, may in certain women be associated with increased risk.[38][39]
Overdose
There have been no reports of serious side effects with overdose of norethisterone, even in small children.[40] As such, overdose usually does not require treatment.[40] High dosages of as much as 60 mg/day norethisterone have been studied for extended treatment durations without serious adverse effects described.[32]
Interactions
5α-Reductase plays an important role in the metabolism of norethisterone, and 5α-reductase inhibitors such as finasteride and dutasteride can inhibit its metabolism.[citation needed] Norethisterone is partially metabolized via hydroxylation by CYP3A4, and inhibitors and inducers of CYP3A4 can significantly alter circulating levels of norethisterone.[1] For instance, the CYP3A4 inducers rifampicin and bosentan have been found to decrease norethisterone exposure by 42% and 23%, respectively, and the CYP3A4 inducers carbamazepine and St. John's wort have also been found to accelerate norethisterone clearance.[1]
Pharmacology
Pharmacodynamics
Norethisterone is a potent progestogen and a weak androgen and estrogen.[4] That is, it is a potent agonist of the progesterone receptor (PR) and a weak agonist of the androgen receptor (AR) and the estrogen receptor (ER).[4] Norethisterone itself has insignificant affinity for the ER; its estrogenic activity is from an active metabolite that is formed in very small amounts, ethinylestradiol (EE), which is a very potent estrogen.[4] Norethisterone and its metabolites have negligible affinity for the glucocorticoid receptor (GR) and mineralocorticoid receptor (MR) and hence have no glucocorticoid, antiglucocorticoid, mineralocorticoid, or antimineralocorticoid activity.[4]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Norethisterone | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisteronea | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisteronea | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisteronea | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisteronea | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
Norethisterone acetateb | 20 | 5 | 1 | 0 | 0 | ? | ? |
Norethisterone enanthateb | ? | ? | ? | ? | ? | ? | ? |
Noretynodrelb | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
Etynodiolb | 1 | 0 | 11–18 | 0 | ? | ? | ? |
Etynodiol diacetateb | 1 | 0 | 0 | 0 | 0 | ? | ? |
Lynestrenolb | 1 | 1 | 3 | 0 | 0 | ? | ? |
Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Foonotes: a = Metabolite of norethisterone. b = Prodrug of norethisterone and/or other active metabolites. Sources: [4][41][42][43][44] | |||||||
Progestogenic activity
Norethisterone displays 8 times higher progestational activity than progesterone.[45] It is also a potent progestogen and binds to the PR with approximately 150% of the affinity of progesterone.[4] In contrast, its parent compounds, testosterone, nandrolone (19-nortestosterone), and ethisterone (17α-ethynyltestosterone), have 2%, 22%, and 44% of the relative binding affinity of progesterone for the PR.[46] Unlike norethisterone, its major active metabolite 5α-dihydronorethisterone (5α-DHNET), which is formed by 5α-reductase, has been found to possess both progestogenic and marked antiprogestogenic activity,[47] although its affinity for the PR is greatly reduced relative to norethisterone at only 25% of that of progesterone.[4] Norethisterone produces similar changes in the endometrium and vagina, such as endometrial transformation, and is similarly antigonadotropic, ovulation-inhibiting, and thermogenic in women compared to progesterone, which is in accordance with its progestogenic activity.[48][46][49]
Androgenic activity
Norethisterone has approximately 15% of the affinity of the anabolic–androgenic steroid (AAS) metribolone (R-1881) for the AR, and in accordance, is weakly androgenic.[4] In contrast to norethisterone, 5α-DHNET, the major metabolite of norethisterone, shows higher affinity for the AR, with approximately 27% of the affinity of metribolone.[4] However, although 5α-DHNET has higher affinity for the AR than norethisterone, it has significantly diminished and in fact almost abolished androgenic potency in comparison to norethisterone in rodent bioassays.[50][51] Similar findings were observed for ethisterone (17α-ethynyltestosterone) and its 5α-reduced metabolite, whereas 5α-reduction enhanced both the AR affinity and androgenic potency of testosterone and nandrolone (19-nortestosterone) in rodent bioassays.[51] As such, it appears that the ethynyl group of norethisterone at the C17α position is responsible for its loss of androgenicity upon 5α-reduction.[51]
Norethisterone (0.5 to 3 mg/day) has been found to dose-dependently decrease circulating SHBG levels, which is a common property of androgens and is due to AR-mediated suppression of hepatic SHBG production.[31] The drug also has estrogenic activity, and estrogens are known to increase SHBG hepatic production and circulating levels, so it would appear that the androgenic activity of norethisterone overpowers its estrogenic activity in this regard.[31]
Norethisterone is bound to a considerable extent (36%) to SHBG in circulation.[4] Although it has lower affinity for SHBG than endogenous androgens and estrogens,[52] Norethisterone may displace testosterone from SHBG and thereby increase free testosterone levels, and this action may contribute to its weak androgenic effects.[53]
Estrogenic activity

Ethinylestradiol (EE), the metabolite of norethisterone responsible for its estrogenic activity.[4]
Norethisterone binds to the ERs, the ERα and the ERβ, with 0.07% and 0.01% of the relative binding affinity of estradiol.[54] Due to these very low relative affinities, it is essentially inactive itself as a ligand of the ERs at clinical concentrations.[4] However, norethisterone has been found to be a substrate for aromatase and is converted in the liver to a small extent (0.35%) to the highly potent estrogen EE, and for this reason, unlike most other progestins, norethisterone has some estrogenic activity.[4] However, with typical dosages of norethisterone used in oral contraceptives (0.5 to 1 mg), the levels of EE produced are low, and it has been said that they are probably without clinical relevance.[4] Conversely, doses of 5 and 10 mg of norethisterone, which are used in the treatment of gynecological disorders, are converted at rates of 0.7% and 1.0% and produce levels of EE that correspond to those produced by 30 and 60 μg dosages of EE, respectively.[2][4] The levels of EE formed by 0.5 and 1 mg of norethisterone have been estimated based on higher dosages as corresponding to 2 and 10 μg dosages of EE, respectively.[2]
Neurosteroid activity
Like progesterone and testosterone, norethisterone is metabolized into 3,5-tetrahydro metabolites.[55] Whether these metabolites of norethisterone interact with the GABAA receptor similarly to the 3,5-tetrahydro metabolites of progesterone and testosterone like allopregnanolone and 3α-androstanediol, respectively, is a topic that does not appear to have been studied and hence requires clarification.[55]
Steroidogenesis inhibition
Norethisterone is a substrate for and is known to be an inhibitor of 5α-reductase, with 4.4% and 20.1% inhibition at 0.1 and 1 μM, respectively.[4] However, therapeutic concentrations of norethisterone are in the low nanomolar range, so this action may not be clinically relevant at typical dosages.[4]
Norethisterone and its major active metabolite 5α-DHNET have been found to act as irreversible aromatase inhibitors (Ki = 1.7 μM and 9.0 μM, respectively).[56] However, like the case of 5α-reductase, the concentrations required are probably too high to be clinically relevant at typical dosages.[4] 5α-DHNET specifically has been assessed and found to be selective in its inhibition of aromatase, and does not affect cholesterol side-chain cleavage enzyme (P450scc), 17α-hydroxylase/17,20-lyase, 21-hydroxylase, or 11β-hydroxylase.[56] Since it is not aromatized (and hence cannot be transformed into an estrogenic metabolite), unlike norethisterone, 5α-DHNET has been proposed as a potential therapeutic agent in the treatment of ER-positive breast cancer.[56]
Other activities
Norethisterone is a very weak inhibitor of CYP2C9 and CYP3A4 (IC50 = 46 μM and 51 μM, respectively), but these actions require very high concentrations of norethisterone that are far above therapeutic circulating levels (which are in the nanomolar range) and hence are probably not clinically relevant.[4]
Norethisterone and some of its 5α-reduced metabolites have been found to produce vasodilating effects in animals that are independent of sex steroid receptors and hence appear to be non-genomic in mechanism.[57]
Pharmacokinetics

Norethisterone and ethinylestradiol levels over 24 hours after a single oral dose of 10 mg NETA in postmenopausal women.[31]

Norethisterone and ethinylestradiol levels over 8 weeks after a single intramuscular injection of 200 mg NETE in premenopausal women.[58]
The pharmacokinetics of norethisterone have been reviewed.[4][59]
Absorption
The oral bioavailability of norethisterone is between 47 and 73%, with a mean oral bioavailability of 64%.[2][3]Micronization has been found to significantly improve the oral bioavailability of norethisterone by increasing intestinal absorption and reducing intestinal metabolism.[4] A single 2 mg oral dose of norethisterone has been found to result in peak circulating levels of the drug of 12 ng/mL (40 nmol/L), whereas a single 1 mg oral dose of norethisterone in combination with 2 mg estradiol resulted in peak levels of norethisterone of 8.5 ng/mL (29 nmol/L) one-hour post-administration.[4]
Distribution
The plasma protein binding of norethisterone is 97%.[4] It is bound 61% bound to albumin and 36% bound to SHBG.[4]
Metabolism
Norethisterone has an elimination half-life of 5.2 to 12.8 hours, with a mean elimination half-life of 8.0 hours.[2] The metabolism of norethisterone is very similar to that of testosterone (and nandrolone) and is mainly via reduction of the Δ4double bond to 5α- and 5β-dihydronorethisterone, which is followed by the reduction of the C3 keto group to the four isomers of 3,5-tetrahydronorethisterone.[4] These transformations are catalyzed by 5α- and 5β-reductase and 3α- and 3β-hydroxysteroid dehydrogenase both in the liver and in extrahepatic tissues such as the pituitary gland, uterus, prostate gland, vagina, and breast.[60] With the exception of 3α,5α- and 3β,5α-tetrahydronorethisterone, which have significant affinity for the ER and are estrogenic to some degree, the 3,5-tetrahydro metabolites of norethisterone are inactive in terms of affinity for sex steroid receptors (specifically, the PR, AR, and ER).[61][62][63] A small amount of norethisterone is also converted by aromatase into EE.[2][4][38] Norethisterone is metabolized in the liver via hydroxylation as well, mainly by CYP3A4.[1] Some conjugation (including glucuronidation and sulfation)[60][64] of norethisterone and its metabolites occurs in spite of steric hindrance by the ethynyl group at C17α.[4] The ethynyl group of norethisterone is preserved in approximately 90% of all of its metabolites.[4]
Elimination
Norethisterone is eliminated 33 to 81% in urine and 35 to 43% in feces.[65]
Chemistry
Norethisterone, also known as 17α-ethynyl-19-nortestosterone or as 17α-ethynylestra-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[66][67] It is specifically a derivative of testosterone in which an ethynyl group has been added at the C17α position and the methyl group at the C19 position has been removed; hence, it is a combined derivative of ethisterone (17α-ethynyltestosterone) and nandrolone (19-nortestosterone).[66][67] These modifications result in increased progestogenic and oral activity and decreased androgenic/anabolic activity.[45]
Derivatives
Norethisterone (NET) is the parent compound of a large group of progestins that includes most of the progestins known as the 19-nortestosterone derivatives.[68] This group is divided by chemical structure into the estranes (derivatives of norethisterone) and the gonanes (18-methylgonanes or 13β-ethylestranes; derivatives of levonorgestrel) and includes the following marketed medications:[69]
|
|
Several of these act as prodrugs of norethisterone, including NETA, NETE, etynodiol diacetate, lynestrenol, and quingestanol acetate.[20][21][22]Noretynodrel may also be a prodrug of norethisterone.[4][2]
Non-17α-ethynylated
19-Nortestosterone (19-NT) progestins which are technically not derivatives of norethisterone (as they do not have a C17α ethynyl group) but are still closely related (with other substitutions at the C17α and/or C16β positions) include the following marketed medications:[66][67]
- The C17α vinyl (ethenyl) derivatives norgesterone (17α-vinyl-δ5(10)-19-NT) and norvinisterone (17α-vinyl-19-NT)
- The C17α allyl derivatives allylestrenol (3-deketo-17α-allyl-19-NT) and altrenogest (17α-allyl-δ9,11-19-NT)
- The C17α alkyl derivative normethandrone (17α-methyl-19-NT)
- The C17α cyanomethyl derivative dienogest (17α-cyanomethyl-δ9-19-NT)
- The C16β ethyl derivative oxendolone (16β-ethyl-19-NT)
Synthesis
Chemical syntheses of norethisterone have been published.[66][59]
Synthesis 1

Norethisterone synthesis:[10][70]
Estradiol 3-methyl ether (1, EME) is partially reduced to the 1,5-diene (2) as also occurs for the first step in the synthesis of nandrolone. Oppenauer oxidation then transforms the C17β hydroxyl group into a ketone functionality (3). This is then reacted with metal acetylide into the corresponding C17α ethynyl compound (4). Hydrolysis of the enol ether under mild conditions leads directly to (5),[70] which appears to be noretynodrel (although Lednicer states that it is "etynodrel" in his book (which may be a synonym etynodiol); etynodrel is with a chlorine atom attached), an orally active progestin. This is the progestogen component of the first oral contraceptive to be offered for sale (i.e., Enovid). Treatment of the ethynyl enol ether with strong acid leads to norethisterone (6).[10]
In practice, these and all other combined oral contraceptives are mixtures of 1 to 2% EE or mestranol and an oral progestin. It has been speculated that the discovery of the necessity of estrogen in addition to progestin for contraceptive efficacy is due to the presence of a small amount of unreduced EME (1) in early batches of 2. This when subjected to oxidation and ethynylation, would of course lead to mestranol (3). In any event, the need for the presence of estrogen in the mixture is now well established experimentally.
Synthesis 2
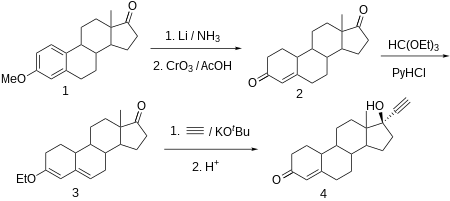
Norethisterone synthesis:[71][72][73][74][75]
Norethisterone is made from estr-4-ene-3,17-dione (bolandione), which in turn is synthesized by partial reduction of the aromatic region of the 3-O-methyl ether of estrone with lithium in liquid ammonia, and simultaneously of the keto group at C17α to a hydroxyl group, which is then oxidized back to a keto group by chromium trioxide in acetic acid. The conjugated C4-C5 olefin and the carbonyl group at C3 is then transformed to dienol ethyl ether using ethyl orthoformate. The obtained product is ethynylated by acetylene in the presence of potassium tert-butoxide. After hydrochloride hydrolysis of the formed O-potassium derivative, during which the enol ether is also hydrolyzed, and the remaining double bond is shifted, the desired norethisterone is obtained.
History
Norethisterone was synthesized for the first time by chemists Luis Miramontes, Carl Djerassi, and George Rosenkranz at Syntex in Mexico City in 1951.[10] It was derived from ethisterone, and was found to possess about 20-fold greater potency as a progestogen in comparison.[76] Norethisterone was the first highly active oral progestogen to be synthesized, and was preceded (as a progestogen) by progesterone (1934), ethisterone (1938), 19-norprogesterone (1944), and 17α-methylprogesterone (1949) as well as by nandrolone (1950), whereas noretynodrel (1952) and norethandrolone (1953) followed the synthesis of norethisterone.[11][12] The drug was first introduced, alone as Norlutin, in the United States in 1957.[13] Norethisterone was subsequently introduced in combination with mestranol as Ortho-Novum in the United States in 1963, and was the second progestin, after noretynodrel in 1960, to be used in an oral contraceptive.[12] In 1964, additional contraceptive preparations containing norethisterone in combination with mestranol or EE, such as Norlestrin and Norinyl, were marketed in the United States.[12]
Society and culture
Generic names
Norethisterone is the INN and BAN of the drug while norethindrone is its USAN.[66][67]
Brand names
Norethisterone (NET), including as NETA and NETE, has been marketed under very large number of brand names throughout the world.[67][18]
| Composition | Dose | Brand names | Use |
|---|---|---|---|
| NET only | Low (e.g., 0.35 mg) | Camila, Errin, Heather, Jencycla, Jolivette, Locilan, Micro-Novum, Micronovum, Micronor, Nor-QD, Nora, Noriday, Ortho Micronor | Progestogen-only oral contraceptive |
| NET or NETA only | High (e.g., 5 mg, 10 mg) | Aygestin, Lupaneta Pack (combination pack with leuprorelin), Norcolut, Norlutate, Primolut N, Primolut Nor, SH-420, Utovlan | Gynecological disorders and other uses |
| NETE only | Injection (e.g., 200 mg) | Depocon, Doryxas, NET-EN, Noristerat, Norigest, Nur-Isterate | Progestogen-only injectable contraceptive |
| NET or NETA with ethinylestradiol | Low (e.g., 0.4 mg, 0.5 mg, 0.75 mg, 1 mg, 1.5 mg) | Aranelle, Balziva, Binovum, Brevicon, Brevinor, Briellyn, Cyclafem, Dasetta, Estrostep, Femcon, Generess, Gildagia, Gildess, Jinteli, Junel, Larin, Leena, Lo Loestrin, Lo Minastrin, Loestrin, Lolo, Lomedia, Microgestin, Minastrin, Modicon, Nelova, Norimin, Norinyl, Nortrel, Ortho, Ortho-Novum, Ovcon, Ovysmen, Philith, Primella, Select, Synphase, Synphasic, Tilia, Tri-Legest, Tri-Norinyl, Trinovum, Vyfemla, Wera, Wymzya, Zenchent, Zeosa | Combined oral contraceptive |
| NET with mestranol | Low (e.g., 1 mg, 2 mg) | Norethin, Noriday, Norinyl, Norquen, Ortho-Novum, Sophia | Combined oral contraceptive |
| NETA with estradiol | Low (e.g., 0.1 mg, 0.5 mg) | Activella, Activelle, Alyacen, Cliane, Climagest, Climesse, Cliovelle, CombiPatch, Elleste Duet, Estalis, Estropause, Eviana, Evorel, Kliane, Kliofem, Kliogest, Kliovance, Mesigyna, Mesygest, Mimvey, Necon, Novofem, Nuvelle, Sequidot, Systen, Trisequens | Combined menopausal hormone therapy |
| NETE with estradiol valerate | Injection (e.g., 50 mg) | Chinese Injectable No. 3, Efectimes, Ginediol, Mesigyna, Mesilar, Meslart, Mesocept, Mesygest, Nofertyl, Nofertyl Lafrancol, Noregyna, Norestrin, Norifam, Norigynon, Nostidyn, Sexseg, Solouna | Combined injectable contraceptive |
Abbreviations: NET = Norethisterone. NETA = Norethisterone acetate. NETE = Norethisterone enanthate. Sources: [77][78][79][80] | |||
Availability
United States
Norethisterone was previously available alone in 5 mg tablets under the brand names Norlutin in the United States, but this formulation has since been discontinued in this country.[81] However, NETA remains available alone in 5 mg tablets under the brand name Aygestin in the United States.[81] It is one of the only non-contraceptive progestogen-only drug formulations that remains available in the United States.[81] The others include progesterone, medroxyprogesterone acetate, megestrol acetate, and hydroxyprogesterone caproate, as well as the atypical agent danazol.[81]
Both norethisterone and NETA are also available in the United States as contraceptives.[81] Norethisterone is available both alone (brand names Camila, Errin, Heather, Micronor, Nor-QD, others) and in combination with EE (Norinyl, Ortho-Novum, others) or mestranol (Norinyl, Ortho-Novum, others), while NETA is available only in combination with EE (Norlestrin, others).[81] NETE is not available in the United States in any form.[81][82][83]
Research
Norethisterone, as NETA and NETE, has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[84]
References
^ abcd Korhonen T, Turpeinen M, Tolonen A, Laine K, Pelkonen O (2008). "Identification of the human cytochrome P450 enzymes involved in the in vitro biotransformation of lynestrenol and norethindrone". J. Steroid Biochem. Mol. Biol. 110 (1–2): 56–66. doi:10.1016/j.jsbmb.2007.09.025. PMID 18356043..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output q{quotes:"""""""'""'"}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ abcdefgh Stanczyk, Frank Z. (Sep 2002). "Pharmacokinetics and Potency of Progestins used for Hormone Replacement Therapy and Contraception" (PDF). Reviews in Endocrine and Metabolic Disorders. 3 (3): 211–224. doi:10.1023/A:1020072325818. ISSN 1389-9155. PMID 12215716.
^ ab Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
^ abcdefghijklmnopqrstuvwxyzaaabacadaeafagahaiajak Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
^ abcd Taitel HF, Kafrissen ME (1995). "Norethindrone--a review of therapeutic applications". Int J Fertil Menopausal Stud. 40 (4): 207–23. PMID 8520623.
^ Kathryn Rhodes Alden; Deitra Leonard Lowdermilk; Mary Catherine Cashion; Shannon E. Perry (27 December 2013). Maternity and Women's Health Care - E-Book. Elsevier Health Sciences. pp. 135–. ISBN 978-0-323-29368-6.
^ https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/018405s023lbl.pdf
^ abcdefg JACOBSON BD (1962). "Hazards of norethindrone therapy during pregnancy". Am. J. Obstet. Gynecol. 84 (7): 962–8. doi:10.1016/0002-9378(62)90075-3. PMID 14450719.
^ abcd IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 417–. ISBN 978-92-832-1291-1.Norethisterone and its acetate and enanthate esters are progestogens that have weak estrogenic and androgenic properties.
^ abcd Djerassi, Carl; Miramontes, L.; Rosenkranz, G.; Sondheimer, Franz (1954). "Steroids. LIV.1Synthesis of 19-Nov-17α-ethynyltestosterone and 19-Nor-17α-methyltestosterone2". Journal of the American Chemical Society. 76 (16): 4092–4094. doi:10.1021/ja01645a010. ISSN 0002-7863.
^ ab Donna Shoupe (7 November 2007). The Handbook of Contraception: A Guide for Practical Management. Springer Science & Business Media. pp. 15–. ISBN 978-1-59745-150-5.
^ abcde Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 74, 76. ISBN 978-0-300-16791-7.
^ ab William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
^ Robert Anthony Hatcher; Anita L. Nelson, M.D. (2007). Contraceptive Technology. Ardent Media. pp. 195–. ISBN 978-1-59708-001-9.
^ Sulochana Gunasheela (14 March 2011). Practical Management of Gynecological Problems. JP Medical Ltd. pp. 31–. ISBN 978-93-5025-240-6.
^ Grimes DA, Lopez LM, O'Brien PA, Raymond EG (2013). "Progestin-only pills for contraception". Cochrane Database Syst Rev (11): CD007541. doi:10.1002/14651858.CD007541.pub3. PMID 24226383.
^ Hussain SF (2004). "Progestogen-only pills and high blood pressure: is there an association? A literature review". Contraception. 69 (2): 89–97. doi:10.1016/j.contraception.2003.09.002. PMID 14759612.
^ ab "Norethisterone".
^ "Generic Aygestin Availability".
^ ab Hammerstein J (1990). "Prodrugs: advantage or disadvantage?". Am. J. Obstet. Gynecol. 163 (6 Pt 2): 2198–203. PMID 2256526.
^ ab Edelman AB, Cherala G, Stanczyk FZ (2010). "Metabolism and pharmacokinetics of contraceptive steroids in obese women: a review". Contraception. 82 (4): 314–23. doi:10.1016/j.contraception.2010.04.016. PMID 20851224.
^ ab Raynaud JP, Ojasoo T (1986). "The design and use of sex-steroid antagonists". J. Steroid Biochem. 25 (5B): 811–33. PMID 3543501.Similar androgenic potential is inherent to norethisterone and its prodrugs (norethisterone acetate, ethynodiol diacetate, lynestrenol, norethynodrel, quingestanol [acetate]).
^ Kamischke, A.; Venherm, S.; Plöger, D.; von Eckardstein, S.; Nieschlag, E. Intramuscular Testosterone Undecanoate and Norethisterone Enanthate in a Clinical Trial for Male Contraception1. The Journal of Clinical Endocrinology & Metabolism 2001, 86, 303-309.
^ Kim, J. J.; Kurita, T.; Bulun, S. E. Progesterone Action in Endometrial Cancer, Endometriosis, Uterine Fibroids, and Breast Cancer. Endocr. Rev. 2012, 34, 130-162.
^ abcde Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 217, 253, 275. ISBN 978-0-08-093292-7.
^ Hägglund H, Remberger M, Klaesson S, Lönnqvist B, Ljungman P, Ringdén O (1998). "Norethisterone treatment, a major risk-factor for veno-occlusive disease in the liver after allogeneic bone marrow transplantation". Blood. 92 (12): 4568–72. PMID 9845522.
^ ab Junkins-Hopkins JM (2010). "Hormone therapy for acne". J. Am. Acad. Dermatol. 62 (3): 486–8. doi:10.1016/j.jaad.2009.12.002. PMID 20159314.
^ ab Arowojolu, Ayodele O.; Gallo, Maria F.; Lopez, Laureen M.; Grimes, David A. (2012-07-11). "Combined oral contraceptive pills for treatment of acne". The Cochrane Database of Systematic Reviews (7): CD004425. doi:10.1002/14651858.CD004425.pub6. ISSN 1469-493X. PMID 22786490.
^ van Vloten WA, Sigurdsson V (2004). "Selecting an oral contraceptive agent for the treatment of acne in women". Am J Clin Dermatol. 5 (6): 435–41. doi:10.2165/00128071-200405060-00008. PMID 15663340.
^ ab Pochi PE, Strauss JS (1965). "Lack of androgen effect on human sebaceous glands with low-dosage norethindrone". Am. J. Obstet. Gynecol. 93 (7): 1002–4. doi:10.1016/0002-9378(65)90162-6. PMID 5843402.
^ abcd Kuhnz W, Heuner A, Hümpel M, Seifert W, Michaelis K (1997). "In vivo conversion of norethisterone and norethisterone acetate to ethinyl etradiol in postmenopausal women". Contraception. 56 (6): 379–85. doi:10.1016/s0010-7824(97)00174-1. PMID 9494772.[...] it has been shown that the repeated oral administration of NET at doses of 0.5 to 3.0 mg to fertile women caused a dose related decrease in the serum levels of SHBG.24 It should be borne in mind that, besides its progestational activity, NET is also characterized by a marked androgenic partial activity, which has a suppressive effect on the synthesis of SHBG and therefore compensates the effects of an additional exposure to EE, on the liver.
^ ab Curwen, S. (1962). "Virilization with Norethisterone". BMJ. 1 (5289): 1415. doi:10.1136/bmj.1.5289.1415-a. ISSN 0959-8138. PMC 1958463.
^ abcd Roxburgh DR, West MJ (1973). "The use of norethisterone to suppress menstruation in the intellectually severely retarded woman". Medical Journal of Australia. 2: 310–313.
^ abc Roxburgh, D. R.; West, M. J. (1974). "The use of norethisterone to suppress menstruation in the intellectually severely retarded woman". Obstetrical & Gynecological Survey. 29 (8): 564. doi:10.1097/00006254-197408000-00021. ISSN 0029-7828.
^ Board JA (1965). "Clinical Evaluation of the Oral Contraceptive Use of Norethindrone 5 mg. plus Mestranol 0.075 mg". Can Med Assoc J. 92: 814–7. PMC 1927985. PMID 14272499.
^ Kaser DJ, Missmer SA, Berry KF, Laufer MR (2012). "Use of norethindrone acetate alone for postoperative suppression of endometriosis symptoms". J Pediatr Adolesc Gynecol. 25 (2): 105–8. doi:10.1016/j.jpag.2011.09.013. PMID 22154396.
^ PAULSEN CA, LEACH RB, LANMAN J, GOLDSTON N, MADDOCK WO, HELLER CG (1962). "Inherent estrogenicity of norethindrone and norethynodrel: comparison with other synthetic progestins and progesterone". J. Clin. Endocrinol. Metab. 22 (10): 1033–9. doi:10.1210/jcem-22-10-1033. PMID 13942007.
^ ab Chu MC, Zhang X, Gentzschein E, Stanczyk FZ, Lobo RA (2007). "Formation of ethinyl estradiol in women during treatment with norethindrone acetate". J. Clin. Endocrinol. Metab. 92 (6): 2205–7. doi:10.1210/jc.2007-0044. PMID 17341557.
^ Anne Connolly; Amanda Britton (31 March 2017). Women's Health in Primary Care. Cambridge University Press. pp. 153–. ISBN 978-1-108-16595-2.
^ ab https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/016954s106lbl.pdf
^ Kuhl H (September 1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-O. PMID 2170822.
^ Philibert D, Bouchoux F, Degryse M, Lecaque D, Petit F, Gaillard M (October 1999). "The pharmacological profile of a novel norpregnance progestin (trimegestone)". Gynecol. Endocrinol. 13 (5): 316–26. doi:10.3109/09513599909167574. PMID 10599548.
^ Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids": 169–214. doi:10.1016/B978-0-12-060308-4.50010-X.
^ Pugeat MM, Dunn JF, Nisula BC (July 1981). "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. doi:10.1210/jcem-53-1-69. PMID 7195405.
^ ab Chwalisz K, Surrey E, Stanczyk FZ (2012). "The Hormonal Profile of Norethindrone Acetate: Rationale for Add-Back Therapy With Gonadotropin-Releasing Hormone Agonists in Women With Endometriosis". Reprod Sci. 19 (6): 563–571. doi:10.1177/1933719112438061. PMID 22457429.
^ ab Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
^ Chu YH, Li QA, Zhao ZF, Zhou YP, Cao DC (1985). "[Antiprogestational action of 5 alpha-dihydronorethisterone]". Zhongguo Yao Li Xue Bao (in Chinese). 6 (2): 125–9. PMID 2934946.
^ Linda E. McCuistion; Joyce LeFever Kee; Evelyn R. Hayes (25 March 2014). Pharmacology: A Patient-Centered Nursing Process Approach. Elsevier Health Sciences. pp. 846–. ISBN 978-0-323-29348-8.
^ GREENBLATT RB (1956). "The progestational activity of 17-alpha-ethinyl-19-nortestosterone". J. Clin. Endocrinol. Metab. 16 (7): 869–75. doi:10.1210/jcem-16-7-869. PMID 13332050.
^ Fragkaki AG, Angelis YS, Koupparis M, Tsantili-Kakoulidou A, Kokotos G, Georgakopoulos C (2009). "Structural characteristics of anabolic androgenic steroids contributing to binding to the androgen receptor and to their anabolic and androgenic activities. Applied modifications in the steroidal structure". Steroids. 74 (2): 172–97. doi:10.1016/j.steroids.2008.10.016. PMID 19028512.Many synthetic steroids with high myotrophic activity exhibit myotrophic–androgenic dissociation, since, due to changes introduced in the structure of ring A, they will probably not be substrates for the 5α-reductases [85]. 5α-Reduction does not always amplify the androgenic potency in spite of high RBA of androgens to the AR. This is the case for norethisterone (Fig. 1, 34), a synthetic 19-nor-17α-ethynyl testosterone derivative, which also undergoes enzyme-mediated 5α-reduction and exerts potent androgenic effects in target organs. 5α-Reduced norethisterone displays a higher AR binding but shows a significantly lower androgenic potency than unchanged norethisterone [102,103].
^ abc Lemus AE, Enríquez J, García GA, Grillasca I, Pérez-Palacios G (1997). "5alpha-reduction of norethisterone enhances its binding affinity for androgen receptors but diminishes its androgenic potency". J. Steroid Biochem. Mol. Biol. 60 (1–2): 121–9. doi:10.1016/s0960-0760(96)00172-0. PMID 9182866.
^ Marcus Filshie; John Guillebaud (22 October 2013). Contraception: Science and Practice. Elsevier Science. pp. 26–. ISBN 978-1-4831-6366-6.Norethisterone binds to SHBG with less affinity than endogenous androgens and oestrogens [...]
^ Ricardo Azziz (8 November 2007). Androgen Excess Disorders in Women. Springer Science & Business Media. pp. 124–. ISBN 978-1-59745-179-6.
^ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
^ ab Giatti, Silvia; Melcangi, Roberto Cosimo; Pesaresi, Marzia (2016). "The other side of progestins: effects in the brain". Journal of Molecular Endocrinology. 57 (2): R109–R126. doi:10.1530/JME-16-0061. ISSN 0952-5041.
^ abc Yamamoto T, Tamura T, Kitawaki J, Osawa Y, Okada H (1994). "Suicide inactivation of aromatase in human placenta and uterine leiomyoma by 5 alpha-dihydronorethindrone, a metabolite of norethindrone, and its effect on steroid-producing enzymes". Eur. J. Endocrinol. 130 (6): 634–40. doi:10.1530/eje.0.1300634. PMID 8205267.
^ Perusquía M, Villalón CM, Navarrete E, García GA, Pérez-Palacios G, Lemus AE (2003). "Vasodilating effect of norethisterone and its 5 alpha metabolites: a novel nongenomic action". Eur. J. Pharmacol. 475 (1–3): 161–9. doi:10.1016/s0014-2999(03)02106-x. PMID 12954372.
^ Friedrich C, Berse M, Klein S, Rohde B, Höchel J (June 2018). "In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate". J Clin Pharmacol. 58 (6): 781–789. doi:10.1002/jcph.1079. PMID 29522253.
^ ab Die Gestagene. Springer-Verlag. 27 November 2013. pp. 13–14, 283–284. ISBN 978-3-642-99941-3.
^ ab Schoonen WG, Deckers GH, de Gooijer ME, de Ries R, Kloosterboer HJ (2000). "Hormonal properties of norethisterone, 7α-methyl-norethisterone and their derivatives". J. Steroid Biochem. Mol. Biol. 74 (4): 213–22. doi:10.1016/s0960-0760(00)00125-4. PMID 11162927.[...] several mono- and disulphated as well as mono- and diglucuronidated metabolites of NET have been detected in urine from NET treated women [16,17]. In unconjugated form these NET (or MeNET) metabolites are represented by 5α- and 5β-reduced NET (5α-NET or 5β-NET) and by 3α- and 3β-hydrogenated 5α-NET and 5β-NET, leading to 3α,5α-NET, 3β,5α-NET, 3α,5β-NET and 3β,5β-NET or their corresponding MeNET metabolites (Figs. 1 and 2). These steroid conversions of NET or MeNET may take place in the liver, but also in the pituitary, endometrium, prostate, vagina and breast. The enzymes involved in these metabolic processes are 5α- and 5β-reductase as well as 3α- and 3β-hydroxysteroid dehydrogenase (HSD).
^ Chávez BA, Vilchis F, Pérez AE, García GA, Grillasca I, Pérez-Palacios G (1985). "Stereospecificity of the intracellular binding of norethisterone and its A-ring reduced metabolites". J. Steroid Biochem. 22 (1): 121–6. doi:10.1016/0022-4731(85)90151-7. PMID 3871879.
^ Garza-Flores J, Vilchis F, García GA, Menjívar M, Pérez-Palacios G (1986). "A-ring reduction enhances the antigonadotropic potency of norethisterone". Acta Endocrinol. 112 (2): 278–83. doi:10.1530/acta.0.1120278. PMID 3090814.
^ Lemus AE, Enríquez J, Hernández A, Santillán R, Pérez-Palacios G (2009). "Bioconversion of norethisterone, a progesterone receptor agonist into estrogen receptor agonists in osteoblastic cells". J. Endocrinol. 200 (2): 199–206. doi:10.1677/JOE-08-0166. PMID 19008332.
^ Scarsi, Kimberly K.; Darin, Kristin M.; Chappell, Catherine A.; Nitz, Stephanie M.; Lamorde, Mohammed (2016). "Drug–Drug Interactions, Effectiveness, and Safety of Hormonal Contraceptives in Women Living with HIV". Drug Safety. 39 (11): 1053–1072. doi:10.1007/s40264-016-0452-7. ISSN 0114-5916. PMC 5048570. PMID 27562873.
^ Leslie J. DeGroot (2001). Endocrinology. W.B. Saunders Co. p. 2617. ISBN 978-0-7216-7843-6.
^ abcde J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 886–. ISBN 978-1-4757-2085-3.
^ abcde Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 749–. ISBN 978-3-88763-075-1.
^ Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 112–. ISBN 978-1-4612-2730-4.
^ Kenneth J. Ryan (1999). Kistner's Gynecology and Women's Health. Mosby. p. 292. ISBN 978-0-323-00201-1.
^ ab Frank B. Colton, U.S. Patent 2,655,518 (1952 to Searle & Co).
^ Ringold, H. J.; Rosenkranz, G.; Sondheimer, F. (1956). "Steroids. LXXX.11-Methyl-19-nortestosterone and 1-Methyl-17α-ethinyl-19-nortestosterone". Journal of the American Chemical Society. 78 (11): 2477–2479. doi:10.1021/ja01592a037.
^ Ueberwasser, H.; Heusler, K.; Kalvoda, J.; Meystre, C.; Wieland, P.; Anner, G.; Wettstein, A. (1963). "19-Norsteroide II. Ein einfaches Herstellungsverfahren für 19-Norandrostan-Derivate. über Steroide, 193. Mitteilung". Helvetica Chimica Acta. 46: 344–352. doi:10.1002/hlca.19630460135.
^ Onken, D; Heublein, D (1970). "Ethinylated steroids". Die Pharmazie. 25 (1): 3–9. PMID 4914401.
^ U.S. Patent 2,744,122
^ U.S. Patent 2,774,777
^ Regidor PA, Schindler AE (2017). "Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone". Oncotarget. 8 (47): 83334–83342. doi:10.18632/oncotarget.19833. PMC 5669973. PMID 29137347.
^ https://www.drugs.com/international/norethisterone.html
^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 27 November 2016.
^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 749–. ISBN 978-3-88763-075-1.
^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0. Lay summary.
^ abcdefg "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 27 November 2016.
^ Vern L. Bullough (2001). Encyclopedia of Birth Control. ABC-CLIO. pp. 145–. ISBN 978-1-57607-181-6.
^ Ellen H. Moskowitz; Bruce Jennings (13 September 1996). Coerced Contraception?: Moral and Policy Challenges of Long Acting Birth Control. Georgetown University Press. pp. 40–. ISBN 978-1-58901-807-5.
^ Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
Further reading
Brogden RN, Speight TM, Avery GS (1973). "Progestagen-only oral contraceptives: a preliminary report of the action and clinical use of norgestrel and norethisterone". Drugs. 6 (3): 169–81. doi:10.2165/00003495-197306030-00004. PMID 4130566.
"Norethisterone and norethisterone acetate". IARC Monogr Eval Carcinog Risk Chem Hum. 21: 441–60. December 1979. PMID 120838.
Stanczyk FZ, Roy S (July 1990). "Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids". Contraception. 42 (1): 67–96. PMID 2143719.
Wiseman LR, McTavish D (March 1994). "Transdermal estradiol/norethisterone. A review of its pharmacological properties and clinical use in postmenopausal women". Drugs Aging. 4 (3): 238–56. doi:10.2165/00002512-199404030-00006. PMID 8199397.
Taitel HF, Kafrissen ME (1995). "Norethindrone--a review of therapeutic applications". Int J Fertil Menopausal Stud. 40 (4): 207–23. PMID 8520623.
Schoonen WG, Deckers GH, de Gooijer ME, de Ries R, Kloosterboer HJ (2000). "Hormonal properties of norethisterone, 7α-methyl-norethisterone and their derivatives". J. Steroid Biochem. Mol. Biol. 74 (4): 213–22. doi:10.1016/s0960-0760(00)00125-4. PMID 11162927.
Maier WE, Herman JR (August 2001). "Pharmacology and toxicology of ethinyl estradiol and norethindrone acetate in experimental animals". Regul. Toxicol. Pharmacol. 34 (1): 53–61. doi:10.1006/rtph.2001.1483. PMID 11502156.
Riis BJ, Lehmann HJ, Christiansen C (October 2002). "Norethisterone acetate in combination with estrogen: effects on the skeleton and other organs. A review". Am. J. Obstet. Gynecol. 187 (4): 1101–16. PMID 12389012.
Draper BH, Morroni C, Hoffman M, Smit J, Beksinska M, Hapgood J, Van der Merwe L (July 2006). "Depot medroxyprogesterone versus norethisterone oenanthate for long-acting progestogenic contraception". Cochrane Database Syst Rev (3): CD005214. doi:10.1002/14651858.CD005214.pub2. PMID 16856087.
Casey CL, Murray CA (2008). "HT update: spotlight on estradiol/norethindrone acetate combination therapy". Clin Interv Aging. 3 (1): 9–16. PMC 2544373. PMID 18488874.
Rajput R, Dhuan J, Agarwal S, Gahlaut PS (August 2008). "Central venous sinus thrombosis in a young woman taking norethindrone acetate for dysfunctional uterine bleeding: case report and review of literature". J Obstet Gynaecol Can. 30 (8): 680–683. doi:10.1016/S1701-2163(16)32916-4. PMID 18786290.
Chwalisz K, Surrey E, Stanczyk FZ (June 2012), "The hormonal profile of norethindrone acetate: rationale for add-back therapy with gonadotropin-releasing hormone agonists in women with endometriosis", Reprod Sci, 19 (6): 563–71, doi:10.1177/1933719112438061, PMID 22457429
Levine S, Muneyyirci-Delale, O (2017), Norethindrone Acetate: Pharmacokinetics, Potency and Alternative Clinical Applications (PDF), MedCrave