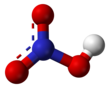
Nitric acid
 | |||
| |||
| Names | |||
|---|---|---|---|
IUPAC name Nitric acid | |||
| Other names Aqua fortis, Spirit of niter, Eau forte, Hydrogen nitrate, Acidum nitricum | |||
| Identifiers | |||
CAS Number |
| ||
3D model (JSmol) |
| ||
| 3DMet | B00068 | ||
ChEBI |
| ||
ChEMBL |
| ||
ChemSpider |
| ||
ECHA InfoCard | 100.028.832 | ||
EC Number | 231-714-2 | ||
Gmelin Reference | 1576 | ||
KEGG |
| ||
MeSH | Nitric+acid | ||
PubChem CID |
| ||
RTECS number | QU5775000 | ||
UNII |
| ||
UN number | 2031 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula | HNO3 | ||
Molar mass | 7001630120000000000♠63.012 g·mol−1 | ||
| Appearance | Colorless, yellow or red fuming liquid[1] | ||
Odor | acrid, suffocating[1] | ||
Density | 1.51 g cm−3, 1.41 g cm−3 [68% w/w] | ||
Melting point | −42 °C (−44 °F; 231 K) | ||
Boiling point | 83 °C (181 °F; 356 K) 68% solution boils at 121 °C (250 °F; 394 K) | ||
Solubility in water | Completely miscible | ||
log P | −0.13[2] | ||
Vapor pressure | 48 mmHg (20 °C)[1] | ||
Acidity (pKa) | −1.4[3] | ||
Conjugate base | Nitrate | ||
Magnetic susceptibility (χ) | 3004801000000000000♠−1.99×10−5 cm3/mol | ||
Refractive index (nD) | 1.397 (16.5 °C) | ||
Dipole moment | 2.17 ± 0.02 D | ||
| Thermochemistry | |||
Std molar entropy (S | 146 J·mol−1·K−1[4] | ||
Std enthalpy of formation (ΔfH | −207 kJ·mol−1[4] | ||
| Hazards | |||
Safety data sheet | ICSC 0183 PCTL Safety Website | ||
GHS pictograms |     | ||
GHS signal word | DANGER | ||
GHS hazard statements | H272, H300, H310, H330, H373, H411 | ||
GHS precautionary statements | P210, P220, P260, P305+351+338, P310, P370+378 | ||
NFPA 704 |  0 4 2 OX | ||
Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) | 138 ppm (rat, 30 min)[1] | ||
| US health exposure limits (NIOSH): | |||
PEL (Permissible) | TWA 2 ppm (5 mg/m3)[1] | ||
REL (Recommended) | TWA 2 ppm (5 mg/m3) ST 4 ppm (10 mg/m3)[1] | ||
IDLH (Immediate danger) | 25 ppm[1] | ||
| Related compounds | |||
Other anions | Nitrous acid | ||
Other cations | Sodium nitrate Potassium nitrate Ammonium nitrate | ||
Related compounds | Dinitrogen pentoxide | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Infobox references | |||
Nitric acid (HNO3), also known as aqua fortis (Latin for "strong water") and spirit of niter, is a highly corrosive mineral acid.
The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as white fuming nitric acid at concentrations above 95%, or red fuming nitric acid at concentrations above 86%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
Contents
1 Physical and chemical properties
1.1 Contamination with nitrogen dioxide
1.2 Fuming nitric acid
1.3 Anhydrous nitric acid
1.4 Structure and bonding
2 Reactions
2.1 Acid-base properties
2.2 Reactions with metals
2.3 Reactions with non-metals
2.4 Xanthoproteic test
3 Production
3.1 Laboratory synthesis
4 Uses
4.1 Precursor to organic nitrogen compounds
4.2 Use as an oxidant
4.3 Rocket propellant
4.4 Niche uses
4.4.1 Analytical reagent
4.4.2 Woodworking
4.4.3 Etchant and cleaning agent
5 Safety
6 History
7 References
8 External links
Physical and chemical properties
Commercially available nitric acid is an azeotrope with water at a concentration of 68% HNO3, which is the ordinary concentrated nitric acid of commerce. This solution has a boiling temperature of 120.5 °C at 1 atm. Two solid hydrates are known; the monohydrate (HNO3·H2O or [H3O]NO3) and the trihydrate (HNO3·3H2O).

Nitric acid 70%
Nitric acid of commercial interest usually consists of the maximum boiling azeotrope of nitric acid and water, which is approximately 68% HNO3, (approx. 15 molar). The density of concentrated nitric acid is 1.35 g/cm3 (68% conc). This is considered concentrated or technical grade, while reagent grades are specified at 70% HNO3. An older density scale is occasionally seen, with concentrated nitric acid specified as 42° Baumé.[5]
Contamination with nitrogen dioxide

Fuming nitric acid contaminated with yellow nitrogen dioxide.
Nitric acid is subject to thermal or light decomposition and for this reason it was often stored in brown glass bottles:
- 4 HNO3 → 2 H2O + 4 NO2 + O2
This reaction may give rise to some non-negligible variations in the vapor pressure above the liquid because the nitrogen oxides produced dissolve partly or completely in the acid.
The nitrogen dioxide (NO2) remains dissolved in the nitric acid coloring it yellow or even red at higher temperatures. While the pure acid tends to give off white fumes when exposed to air, acid with dissolved nitrogen dioxide gives off reddish-brown vapors, leading to the common name "red fuming acid" or "fuming nitric acid" – the most concentrated form of nitric acid at Standard Temperature and Pressure (STP). Nitrogen oxides (NOx) are soluble in nitric acid.
Fuming nitric acid
A commercial grade of fuming nitric acid contains 98% HNO3 and has a density of 1.50 g/cm3. This grade is often used in the explosives industry. It is not as volatile nor as corrosive as the anhydrous acid and has the approximate concentration of 21.4 M.
Red fuming nitric acid, or RFNA, contains substantial quantities of dissolved nitrogen dioxide (NO2) leaving the solution with a reddish-brown color. Due to the dissolved nitrogen dioxide, the density of red fuming nitric acid is lower at 1.490 g/cm3.
An inhibited fuming nitric acid (either IWFNA, or IRFNA) can be made by the addition of 0.6 to 0.7% hydrogen fluoride (HF). This fluoride is added for corrosion resistance in metal tanks. The fluoride creates a metal fluoride layer that protects the metal.
Anhydrous nitric acid
White fuming nitric acid, pure nitric acid or WFNA, is very close to anhydrous nitric acid. It is available as 99.9% nitric acid by assay. One specification for white fuming nitric acid is that it has a maximum of 2% water and a maximum of 0.5% dissolved NO2. Anhydrous nitric acid has a density of 1.513 g/cm3 and has the approximate concentration of 24 molar. Anhydrous nitric acid is a colorless mobile liquid with a density of 1.512 g/cm3, which solidifies at −42 °C to form white crystals. As it decomposes to NO2 and water, it obtains a yellow tint. It boils at 83 °C. It is usually stored in a glass shatterproof amber bottle with twice the volume of head space to allow for pressure build up, but even with those precautions the bottle must be vented monthly to release pressure.
Structure and bonding
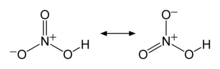
Two major resonance representations of HNO3
The molecule is planar. Two of the N–O bonds are equivalent and relatively short (this can be explained by theories of resonance; the canonical forms show double-bond character in these two bonds, causing them to be shorter than typical N–O bonds), and the third N–O bond is elongated because the O atom is also attached to a proton.[6][7]
Reactions
Acid-base properties
Nitric acid is normally considered to be a strong acid at ambient temperatures. There is some disagreement over the value of the acid dissociation constant, though the pKa value is usually reported as less than −1. This means that the nitric acid in diluted solution is fully dissociated except in extremely acidic solutions. The pKa value rises to 1 at a temperature of 250 °C.[8]
Nitric acid can act as a base with respect to an acid such as sulfuric acid:
- HNO3 + 2 H2SO4 ⇌ NO+
2 + H3O+ + 2 HSO−
4; Equilibrium constant: K ≈ 22
The nitronium ion, NO+
2, is the active reagent in aromatic nitration reactions. Since nitric acid has both acidic and basic properties, it can undergo an autoprotolysis reaction, similar to the self-ionization of water:
- 2 HNO3 ⇌ NO+
2 + NO−
3 + H2O
Reactions with metals
Nitric acid reacts with most metals, but the details depend on the concentration of the acid and the nature of the metal. Dilute nitric acid behaves as a typical acid in its reaction with most metals. Magnesium, manganese, and zinc liberate H2:
- Mg + 2 HNO3 → Mg(NO3)2 + H2 (Magnesium nitrate)
- Mn + 2 HNO3 → Mn(NO3)2 + H2 (Manganese nitrate)
Nitric acid can oxidize non-active metals such as copper and silver. With these non-active or less electropositive metals the products depend on temperature and the acid concentration. For example, copper reacts with dilute nitric acid at ambient temperatures with a 3:8 stoichiometry:
- 3 Cu + 8 HNO3 → 3 Cu2+ + 2 NO + 4 H2O + 6 NO−
3
The nitric oxide produced may react with atmospheric oxygen to give nitrogen dioxide. With more concentrated nitric acid, nitrogen dioxide is produced directly in a reaction with 1:4 stoichiometry:
- Cu + 4 H+ + 2 NO−
3 → Cu2+ + 2 NO2 + 2 H2O
Upon reaction with nitric acid, most metals give the corresponding nitrates. Some metalloids and metals give the oxides; for instance, Sn, As, Sb, and Ti are oxidized into SnO2, As2O5, Sb2O5, and TiO2 respectively.[9]
Some precious metals, such as pure gold and platinum-group metals do not react with nitric acid, though pure gold does react with aqua regia, a mixture of concentrated nitric acid and hydrochloric acid. However, some less noble metals (Ag, Cu, ...) present in some gold alloys relatively poor in gold such as colored gold can be easily oxidized and dissolved by nitric acid, leading to colour changes of the gold-alloy surface. Nitric acid is used as a cheap means in jewelry shops to quickly spot low-gold alloys (< 14 carats) and to rapidly assess the gold purity.
Being a powerful oxidizing agent, nitric acid reacts violently with many non-metallic compounds, and the reactions may be explosive. Depending on the acid concentration, temperature and the reducing agent involved, the end products can be variable. Reaction takes place with all metals except the noble metals series and certain alloys. As a general rule, oxidizing reactions occur primarily with the concentrated acid, favoring the formation of nitrogen dioxide (NO2). However, the powerful oxidizing properties of nitric acid are thermodynamic in nature, but sometimes its oxidation reactions are rather kinetically non-favored. The presence of small amounts of nitrous acid (HNO2) greatly enhance the rate of reaction.[9]
Although chromium (Cr), iron (Fe), and aluminium (Al) readily dissolve in dilute nitric acid, the concentrated acid forms a metal-oxide layer that protects the bulk of the metal from further oxidation. The formation of this protective layer is called passivation. Typical passivation concentrations range from 20% to 50% by volume (see ASTM A967-05). Metals that are passivated by concentrated nitric acid are iron, cobalt, chromium, nickel, and aluminium.[9]
Reactions with non-metals
Being a powerful oxidizing acid, nitric acid reacts violently with many organic materials and the reactions may be explosive. The hydroxyl group will typically strip a hydrogen from the organic molecule to form water, and the remaining nitro group takes the hydrogen's place. Nitration of organic compounds with nitric acid is the primary method of synthesis of many common explosives, such as nitroglycerin and trinitrotoluene (TNT). As very many less stable byproducts are possible, these reactions must be carefully thermally controlled, and the byproducts removed to isolate the desired product.
Reaction with non-metallic elements, with the exceptions of nitrogen, oxygen, noble gases, silicon, and halogens other than iodine, usually oxidizes them to their highest oxidation states as acids with the formation of nitrogen dioxide for concentrated acid and nitric oxide for dilute acid.
- C + 4 HNO3 → CO2 + 4 NO2 + 2 H2O
or
- 3 C + 4 HNO3 → 3 CO2 + 4 NO + 2 H2O
Concentrated nitric acid oxidizes I2, P4, and S8 into HIO3, H3PO4, and H2SO4, respectively.[9]
Xanthoproteic test
Nitric acid reacts with proteins to form yellow nitrated products. This reaction is known as the xanthoproteic reaction. This test is carried out by adding concentrated nitric acid to the substance being tested, and then heating the mixture. If proteins that contain amino acids with aromatic rings are present, the mixture turns yellow. Upon adding a base such as ammonia, the color turns orange. These color changes are caused by nitrated aromatic rings in the protein.[10][11]Xanthoproteic acid is formed when the acid contacts epithelial cells. Respective local skin color changes are indicative of inadequate safety precautions when handling nitric acid.
Production
Nitric acid is made by reaction of nitrogen dioxide (NO2) with water.
- 4 NO2 + 2 H2O → 2 HNO3 + NO + NO2 + H2O
Or, shortened:
- 3 NO2 + H2O → 2 HNO3 + NO
Normally, the nitric oxide produced by the reaction is reoxidized by the oxygen in air to produce additional nitrogen dioxide.
Bubbling nitrogen dioxide through hydrogen peroxide can help to improve acid yield.
- 2 NO2 + H2O2 → 2 HNO3
Commercial grade nitric acid solutions are usually between 52% and 68% nitric acid. Production of nitric acid is via the Ostwald process, named after German chemist Wilhelm Ostwald. In this process, anhydrous ammonia is oxidized to nitric oxide, in the presence of platinum or rhodium gauze catalyst at a high temperature of about 500 K and a pressure of 9 atm.
- 4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (g) (ΔH = −905.2 kJ)
Nitric oxide is then reacted with oxygen in air to form nitrogen dioxide.
- 2 NO (g) + O2 (g) → 2 NO2 (g) (ΔH = −114 kJ/mol)
This is subsequently absorbed in water to form nitric acid and nitric oxide.
- 3 NO2 (g) + H2O (l) → 2 HNO3 (aq) + NO (g) (ΔH = −117 kJ/mol)
The nitric oxide is cycled back for reoxidation. Alternatively, if the last step is carried out in air:
- 4 NO2 (g) + O2 (g) + 2 H2O (l) → 4 HNO3 (aq)
The aqueous HNO3 obtained can be concentrated by distillation up to about 68% by mass. Further concentration to 98% can be achieved by dehydration with concentrated H2SO4. By using ammonia derived from the Haber process, the final product can be produced from nitrogen, hydrogen, and oxygen which are derived from air and natural gas as the sole feedstocks.[12]
Prior to the introduction of the Haber process for the production of ammonia in 1913, nitric acid was produced using the Birkeland–Eyde process, also known as the arc process. This process is based upon the oxidation of atmospheric nitrogen by atmospheric oxygen to nitric oxide at very high temperatures. An electric arc was used to provide the high temperatures, and yields of up to 4% nitric oxide were obtained. The nitric oxide was cooled and oxidized by the remaining atmospheric oxygen to nitrogen dioxide, and this was subsequently absorbed in dilute nitric acid. The process was very energy intensive and was rapidly displaced by the Ostwald process once cheap ammonia became available.
Laboratory synthesis
In laboratory, nitric acid can be made by thermal decomposition of copper(II) nitrate, producing nitrogen dioxide and oxygen gases, which are then passed through water to give nitric acid.
- 2 Cu(NO3)2 → 2 CuO (s) + 4 NO2 (g) + O2 (g)
An alternate route is by reaction of approximately equal masses of any nitrate salt such as sodium nitrate with 96% sulfuric acid (H2SO4), and distilling this mixture at nitric acid's boiling point of 83 °C. A nonvolatile residue of the metal hydrogen sulfate remains in the distillation vessel. The red fuming nitric acid obtained may be converted to the white nitric acid.[7]
NaNO3 + H2SO4 → HNO3 + NaHSO4
The dissolved NOx is readily removed using reduced pressure at room temperature (10–30 minutes at 200 mmHg or 27 kPa) to give white fuming nitric acid. This procedure can also be performed under reduced pressure and temperature in one step in order to produce less nitrogen dioxide gas.[citation needed]
Dilute nitric acid may be concentrated by distillation up to 68% acid, which is a maximum boiling azeotrope containing 32% water. In the laboratory, further concentration involves distillation with either sulfuric acid or magnesium nitrate which act as dehydrating agents. Such distillations must be done with all-glass apparatus at reduced pressure, to prevent decomposition of the acid. Industrially, highly concentrated nitric acid is produced by dissolving additional nitrogen dioxide in 68% nitric acid in an absorption tower.[13] Dissolved nitrogen oxides are either stripped in the case of white fuming nitric acid, or remain in solution to form red fuming nitric acid. More recently, electrochemical means have been developed to produce anhydrous acid from concentrated nitric acid feedstock.[14]
Uses

Nitric acid in a laboratory.
The main industrial use of nitric acid is for the production of fertilizers. Nitric acid is neutralized with ammonia to give ammonium nitrate. This application consumes 75–80% of the 26 million tonnes produced annually (1987). The other main applications are for the production of explosives, nylon precursors, and specialty organic compounds.[15]
Precursor to organic nitrogen compounds
In organic synthesis, industrial and otherwise, the nitro group is a versatile functional group. Most derivatives of aniline are prepared via nitration of aromatic compounds followed by reduction. Nitrations entail combining nitric and sulfuric acids to generate the nitronium ion, which electrophilically reacts with aromatic compounds such as benzene. Many explosives, such as TNT, are prepared this way:
C6H5CH3 + 3 HNO3 → C6H2(NO2)3CH3 + 3 H2O
Either concentrated sulfuric acid or oleum absorbs the excess water.
H2S2O7 + H2O → 2 H2SO4
Use as an oxidant
The precursor to nylon, adipic acid, is produced on a large scale by oxidation of cyclohexanone and cyclohexanol with nitric acid.[15]
Rocket propellant
Nitric acid has been used in various forms as the oxidizer in liquid-fueled rockets. These forms include red fuming nitric acid, white fuming nitric acid, mixtures with sulfuric acid, and these forms with HF inhibitor.[16] IRFNA (inhibited red fuming nitric acid) was one of 3 liquid fuel components for the BOMARC missile.[17]
Niche uses
Analytical reagent
In elemental analysis by ICP-MS, ICP-AES, GFAA, and Flame AA, dilute nitric acid (0.5–5.0%) is used as a matrix compound for determining metal traces in solutions.[18] Ultrapure trace metal grade acid is required for such determination, because small amounts of metal ions could affect the result of the analysis.
It is also typically used in the digestion process of turbid water samples, sludge samples, solid samples as well as other types of unique samples which require elemental analysis via ICP-MS, ICP-OES, ICP-AES, GFAA and flame atomic absorption spectroscopy. Typically these digestions use a 50% solution of the purchased HNO
3 mixed with Type 1 DI Water.
In electrochemistry, nitric acid is used as a chemical doping agent for organic semiconductors, and in purification processes for raw carbon nanotubes.
Woodworking
In a low concentration (approximately 10%), nitric acid is often used to artificially age pine and maple. The color produced is a grey-gold very much like very old wax or oil finished wood (wood finishing).[19]
Etchant and cleaning agent
The corrosive effects of nitric acid are exploited for a number of specialty applications, such as etching in printmaking, pickling stainless steel or cleaning silicon wafers in electronics.[20]
A solution of nitric acid, water and alcohol, Nital, is used for etching of metals to reveal the microstructure. ISO 14104 is one of the standards detailing this well known procedure.
Nitric acid is used either in combination with hydrochloric acid or alone to clean glass cover slips and glass slides for high end microscopy applications. [21] It is also used to clean glass prior to silvering in the production of silver mirrors. [22]
Commercially available aqueous blends of 5–30% nitric acid and 15–40% phosphoric acid are commonly used for cleaning food and dairy equipment primarily to remove precipitated calcium and magnesium compounds (either deposited from the process stream or resulting from the use of hard water during production and cleaning). The phosphoric acid content helps to passivate ferrous alloys against corrosion by the dilute nitric acid.[citation needed]
Nitric acid can be used as a spot test for alkaloids like LSD, giving a variety of colours depending on the alkaloid.[23]
Safety
Nitric acid is a corrosive acid and a powerful oxidizing agent. The major hazard posed by it is chemical burns, as it carries out acid hydrolysis with proteins (amide) and fats (ester), which consequently decomposes living tissue (e.g. skin and flesh). Concentrated nitric acid stains human skin yellow due to its reaction with the keratin. These yellow stains turn orange when neutralized.[24] Systemic effects are unlikely, however, and the substance is not considered a carcinogen or mutagen.[25]
The standard first-aid treatment for acid spills on the skin is, as for other corrosive agents, irrigation with large quantities of water. Washing is continued for at least 10–15 minutes to cool the tissue surrounding the acid burn and to prevent secondary damage. Contaminated clothing is removed immediately and the underlying skin washed thoroughly.
Being a strong oxidizing agent, nitric acid can react with compounds such as cyanides, carbides, or metallic powders explosively and with many organic compounds, such as turpentine, violently and hypergolically (i.e. self-igniting). Hence, it should be stored away from bases and organics.
History
The first mention of nitric acid is in Pseudo-Geber's De Inventione Veritatis, wherein it is obtained by calcining a mixture of niter, alum and blue vitriol. It was again described by Albert the Great in the 13th century and by Ramon Lull, who prepared it by heating niter and clay and called it "eau forte" (aqua fortis).[26]
Glauber devised a process to obtain it by distilling potassium nitrate with sulfuric acid. In 1776 Lavoisier showed that it contained oxygen, and in 1785 Henry Cavendish determined its precise composition and showed that it could be synthesized by passing a stream of electric sparks through moist air.[26]
References
^ abcdefg "NIOSH Pocket Guide to Chemical Hazards #0447". National Institute for Occupational Safety and Health (NIOSH)..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ "nitric acid_msds".
^ Bell, R. P. (1973), The Proton in Chemistry (2nd ed.), Ithaca, NY: Cornell University Press
^ ab Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A22. ISBN 978-0-618-94690-7.
^
Dean, John (1992). Lange's Handbook of Chemistry (14 ed.). McGraw-Hill. pp. 2.79–2.80. ISBN 978-0-07-016194-8.
^ Luzzati, V. (1951). "Structure cristalline de l'acide nitrique anhydre". Acta Crystallographica (in French). 4 (2): 120–131. doi:10.1107/S0365110X51000404.
^ ab Allan, D. R.; Marshall, W. G.; Francis, D. J.; Oswald, I. D. H.; Pulham, C. R.; Spanswick, C. (2010). "The crystal structures of the low-temperature and high-pressure polymorphs of nitric acid" (PDF). Dalton Trans. (Submitted manuscript). 39 (15): 3736–3743. doi:10.1039/B923975H. PMID 20354626.
^ IUPAC SC-Database A comprehensive database of published data on equilibrium constants of metal complexes and ligands
^ abcd Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 15: The group 15 elements". Inorganic Chemistry, 3rd Edition. Pearson. ISBN 978-0-13-175553-6.
^ Sherman, Henry Clapp (2007). Methods of organic analysis. Read Books. p. 315. ISBN 978-1-4086-2802-7.
^ Knowles, Frank (2007). A practical course in agricultural chemistry. Read Books. p. 76. ISBN 978-1-4067-4583-2.
^ Considine, Douglas M., ed. (1974). Chemical and process technology encyclopedia. New York: McGraw-Hill. pp. 769–72. ISBN 978-0-07-012423-3.
^ Urbanski, Tadeusz (1965). Chemistry and technology of explosives. Oxford: Pergamon Press. pp. 85–86. ISBN 978-0-08-010239-9.
^ US 6200456, Harrar, Jackson E.; Roland Quong & Lester P. Rigdon et al., "Large-scale production of anhydrous nitric acid and nitric acid solutions of dinitrogen pentoxide", published April 13, 1987, issued March 13, 2001, assigned to United States Department of Energy
^ ab Thiemann, Michael; Scheibler, Erich; Wiegand, Karl Wilhelm (2000). "Nitric Acid, Nitrous Acid, and Nitrogen Oxides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_293. ISBN 978-3527306732.
^ Clark, John D (1972). Ignition!. Rutgers University Press. ISBN 978-0-8135-0725-5.
^ "BOMARC Summary". BILLONY.COM. Retrieved 2009-05-28.
^ Clesceri, Lenore S.; Greenberg, Arnold E.; Eaton, Andrew D., eds. (1998). Standard methods for the examination of water and wastewater (20th ed.). American Public Health Association, American Water Works Association, Water Environment Federation. ISBN 978-0-87553-235-6.
^ Jewitt, Jeff (1997). Hand-applied finishes. Taunton Press. ISBN 978-1-56158-154-2. Retrieved 2009-05-28.
^ Muraoka, Hisashi (1995) "Silicon wafer cleaning fluid with HNO3, HF, HCl, surfactant, and water" U.S. Patent 5,635,463
^ Zeller, Rolf; Rose, Jack; Jacobson, Kenneth A.; Fischer, Andrew H. (2008-05-01). "Preparation of Slides and Coverslips for Microscopy". Cold Spring Harbor Protocols. 2008 (5): pdb.prot4988. doi:10.1101/pdb.prot4988. ISSN 1559-6095. PMID 21356831.
^ Curtis, Heber D. (February 1911). "METHODS OF SILVERING MIRRORS". Publications of the Astronomical Society of the Pacific. 23 (135): 13. doi:10.1086/122040. ISSN 1538-3873.
^ O’Neal, C. L.; Crouch, D. J.; Fatah, A. A. (2000). "Validation of twelve chemical spot tests for the detection of drugs of abuse". Forensic Science International. 109 (3): 189–201. doi:10.1016/S0379-0738(99)00235-2. PMID 10725655.
^ May, Paul (November 2007). "Nitric acid". Retrieved 2009-05-28.
^ "Nitric acid: Toxicological overview". Health Protection Agency. Retrieved 2011-12-07.
^ ab Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 19 (11th ed.). Cambridge University Press. pp. 711–712.
Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 19 (11th ed.). Cambridge University Press. pp. 711–712.
External links
- NIOSH Pocket Guide to Chemical Hazards
- National Pollutant Inventory – Nitric Acid Fact Sheet
- Calculators: surface tensions, and densities, molarities and molalities of aqueous nitric acid