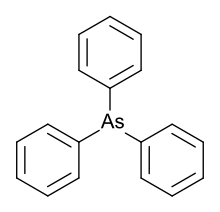
Triphenylarsine
 | |
 | |
| Names | |
|---|---|
IUPAC name Triphenylarsane | |
| Other names Tribenzenidoarsenic Triphenylarsine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
ECHA InfoCard | 100.009.121 |
EC Number | 210-032-9 |
PubChem CID |
|
RTECS number | CH8942500 |
UN number | 3465 |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C18H15As |
Molar mass | 306.24 g·mol−1 |
| Appearance | Colourless solid |
Density | 1.395 g cm−3 |
Melting point | 58 to 61 °C (136 to 142 °F; 331 to 334 K) |
Boiling point | 373 °C (703 °F; 646 K) at 760 mmHg |
Solubility in water | Insoluble |
Solubility | Soluble in ethyl ether, benzene, slightly soluble in ethanol |
Magnetic susceptibility (χ) | -177.0·10−6 cm3/mol |
| Structure | |
Crystal structure | Triclinic |
| Hazards | |
EU classification (DSD) (outdated) | |
R-phrases (outdated) | R23/25, R50/53 |
S-phrases (outdated) | S20/21, S28, S45, S60, S61 |
| Related compounds | |
Related organoarsanes | Trimethylarsine |
Related compounds | Triphenylamine Triphenylborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Triphenylarsine is the chemical compound with the formula As(C6H5)3. This organoarsenic compound, often abbreviated AsPh3, is a colorless crystalline solid that is used as a ligand and a reagent in coordination chemistry and organic synthesis. The molecule is pyramidal with As-C distances of 1.942–1.956 Å and C-As-C angles of 99.6–100.5°.[1]
This compound is prepared by the reaction of arsenic trichloride with chlorobenzene using sodium as the reducing agent:[2]
- AsCl3 + 3 PhCl + 6 Na → AsPh3 + 6 NaCl
Reactions
Reaction of triphenylarsine with lithium gives lithium diphenylarsenide:[3]
- AsPh3 + 2 Li → LiAsPh2 + LiPh
Triphenylarsine is the precursor to tetraphenylarsonium chloride, [AsPh4]Cl, a popular precipitating agent.[2]
AsPh3 forms metal complexes with metals. Most are analogues of the corresponding triphenylphosphine derivatives. Examples include IrCl(CO)(AsPh3)2, RhCl(AsPh3)3, and Fe(CO)4(AsPh3).[4]
References
^ Mazhar-ul-Haque, Hasan A. Tayim, Jamil Ahmed, and William Horne "Crystal and molecular structure of triphenylarsine" Journal of Chemical Crystallography Volume 15, Number 6 / 1985. doi: 10.1007/BF01164771
^ ab Shriner, R. L.; Wolf, C. N. (1963). "Tetraphenylarsonium Chloride Hydrochloride". Organic Syntheses.CS1 maint: Multiple names: authors list (link).mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}; Collective Volume, 4, p. 910
article
^ W. Levason, C. A. Mcauliffe (1976). "cis‐2‐Diphenylarsinovinyldiphenylphosphine and 2‐Diphenylarsinoethyldiphenylphosphine". Inorganic Syntheses. 16: 188–192. doi:10.1002/9780470132470.ch50.CS1 maint: Uses authors parameter (link)
^ C. A. McAuliffe, ed. (1973). Transition Metal Complexes of Phosphorus, Arsenic, and Antimony Ligands. J. Wiley. ISBN 0-470-58117-4.