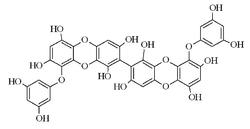
8,8′-Bieckol
 | |
| Names | |
|---|---|
IUPAC name 9-(3,5-dihydroxyphenoxy)-2-[9-(3,5-dihydroxyphenoxy)-1,3,6,8-tetrahydroxydibenzo-p-dioxin-2-yl]dibenzo-p-dioxin-1,3,6,8-tetrol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | C36H22O18 |
Molar mass | 742.55 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
8,8'-Bieckol is an eckol-type phlorotannin found in the brown algae Ecklonia cava[1] and Ecklonia kurome.[2]
References
^ Ahn, M. J.; Yoon, K. D.; Min, S. Y.; Lee, J. S.; Kim, J. H.; Kim, T. G.; Kim, S. H.; Kim, N. G.; Huh, H.; Kim, J. (2004). "Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava". Biological & Pharmaceutical Bulletin. 27 (4): 544–547. doi:10.1248/bpb.27.544. PMID 15056863..mw-parser-output cite.citation{font-style:inherit}.mw-parser-output .citation q{quotes:"""""""'""'"}.mw-parser-output .citation .cs1-lock-free a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .citation .cs1-lock-subscription a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration{color:#555}.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration span{border-bottom:1px dotted;cursor:help}.mw-parser-output .cs1-ws-icon a{background:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center}.mw-parser-output code.cs1-code{color:inherit;background:inherit;border:inherit;padding:inherit}.mw-parser-output .cs1-hidden-error{display:none;font-size:100%}.mw-parser-output .cs1-visible-error{font-size:100%}.mw-parser-output .cs1-maint{display:none;color:#33aa33;margin-left:0.3em}.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-format{font-size:95%}.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-left{padding-left:0.2em}.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-right{padding-right:0.2em}
^ Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Mori, H.; Nakayama, Y.; Takahashi, M. (1989). "Anti-plasmin inhibitor. V. Structures of novel dimeric eckols isolated from the brown alga Ecklonia kurome OKAMURA". Chemical & Pharmaceutical Bulletin. 37 (9): 2438–2440. doi:10.1248/cpb.37.2438. PMID 2605688.
This article about an aromatic compound is a stub. You can help Wikipedia by expanding it. |